Overcoming the leukemia stem cell resistance to intensive chemotherapy has been an area of extensive research over the last two decades. Advances and greater understanding of the molecular biology of leukemia stem cells are in rapid progress. Targeted therapies are currently being used in clinical practice with reasonable response rates, but a cure is being achieved in only a small percentage of patients, most likely due to tumor mutational heterogeneity. A genetically engineered diphtheria toxin fused with interleukin-3 (SL-401 or tagraxofusp) has shown robust activity in blastic plasmacytoid dendritic cell neoplasm and promising response rates in different myeloid malignancies, including eradication of minimal residual disease. Multiple clinical trials are being conducted using this drug and the preliminary results are encouraging.
- SL-401 (tagraxofusp)
- diphtheria immunotoxin
- adverse events
- Myeloid neoplasms
1. Introduction
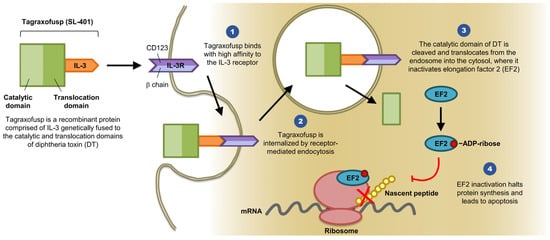
2. Clinical Trials
| SL-401 Clinical Trial Registry Numbers | Primary Malignancy | Status | Study Start Date | Phase | Published Data | Number of Patients | Number of Cycles | Age | Male/Female | Adverse Cytogenetics | Intermediate Cytogenetics | Relapsed (any) and Refractory | Adverse Events | ORR | CR | PR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | R/R or elderly AML and high risk MDS | Completed | NA | I | Leukemia and Lymphoma 2007 | 45 | 1 | 67 (32–81) | 23/22 | 17 | 25 | 35 | Grade III LFTs, Grade II fever, chills, low albumin and hypotension | NA | 1 | 3 |
| NCT00397579 | BPDCN | Completed | May 2013 | I/II | Blood 2014 for the BPDCN | 11 | 1 | 70 (40–77) | 11/0 | NA | NA | 7 | Grade IV thrombocytopenia, Grade III LFTs and neutropenia | NA | 5 | 0 |
| NA | RR/AML and BPDCN | NA | NA | NA | ASH 2015 | 17 | multiple | 63 | NA | NA | NA | NA | Grade V CLS, Grade IV CLS, Grade III LFTs | NA | 4 | NA |
| NCT02270463 | Consolidation Rx in adverse risk AML CR1 | Recruiting | February 2015 | I/II | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NCT02268253 | Advanced high risk MNP (SM, PED, MF, CMML) | Recruiting | December 2014 | I/II | ASH 2016 | 19 | multiple | 69 (42–81) | NA | NA | NA | 19 | Grade III thrombocytopenia and anemia | NA | 1 | NA |
| NCT03113643 | With AZA for Rx naïve AML/high risk MDS not eligible for standard Rx | Recruiting | June 2017 | I | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NCT02661022 | R/R MM | Recruiting | January 2016 | I/II | ASH 2016 | 2 | multiple | 65 (63–67) | NA | NA | NA | 2 | Grade II thrombocytopenia and hypoalbuminemia | NA | 0 | 2 |
| NCT02113982 | BPDCN and R/R AML | Recruiting | September 2014 | I/II | ASCO 2016 (only BPDCN) | 18 | multiple | 70 (45–82) | NA | NA | NA | 10 | CLS and thrombocytopenia | 87% | 8 | NA |
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines7010006
References
- Kayser, S.; Levis, M.J. Advances in targeted therapy for acute myeloid leukaemia. Br. J. Haematol. 2018, 180, 484–500.
- Testa, U.; Riccioni, R.; Diverio, D.; Rossini, A.; Lo Coco, F.; Peschle, C. Interleukin-3 receptor in acute leukemia. Leukemia 2004, 18, 219.
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293.
- Hertler, A.A.; Frankel, A.E. Immunotoxins: a clinical review of their use in the treatment of malignancies. J. Clin. Oncol. 1989, 7, 1932–1942.
- Aruna, G. Immunotoxins: A review of their use in cancer treatment. J. Stem Cells Regen. Med. 2006, 1, 31–36.
- Murphy, J.R. Corynebacterium Diphtheriae. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996.
- Murphy, J.R. Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process. Toxins 2011, 3, 294–308.
- Ruella, M.; Klichinsky, M.; Kenderian, S.S.; Shestova, O.; Ziober, A.; Kraft, D.O.; Feldman, M.; Wasik, M.A.; June, C.H.; Gill, S. Overcoming the immunosuppressive tumor microenvironment of hodgkin lymphoma using chimeric antigen receptor t cells. Cancer Discov. 2017, 7, 1154–1167.
- Apisarnthanarax, N.; Talpur, R.; Duvic, M. Treatment of cutaneous t cell lymphoma: Current status and future directions. Am. J. Clin. Dermatol. 2002, 3, 193–215.
- Feuring-Buske, M.; Frankel, A.E.; Alexander, R.L.; Gerhard, B.; Hogge, D.E. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002, 62, 1730–1736.
- Pemmaraju, N.; Sweet, K.L.; Lane, A.A.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Aung, P.; et al. Results of pivotal phase 2 trial of SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood 2017, 130 (Suppl. S1), 1298.
- Suda, T.; Suda, J.; Ogawa, M.; Ihle, J.N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J. Cell. Physiol. 1985, 124, 182–190.
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Medeiros, B.C.; Carraway, H.E.; Frankfurt, O.; Forman, S.J.; Yang, X.A.; Konopleva, M.; Garnache-Ottou, F.; et al. Clinical and preclinical activity of SL-401, a targeted therapy directed to the interleukin-3 receptor on cancer stem cells and tumor bulk of hematologic malignancies, against blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood 2013, 31 (Suppl. S15), TPS3105.
- Urieto, J.O.; Liu, T.F.; Black, J.H.; Cohen, K.A.; Hall, P.D.; Willingham, M.C.; Penell, L.K.; Hogge, D.E.; Kreitman, R.J.; Frankel, A.E. Expression and purification of the recombinant diphtheria fusion toxin DT388IL3 for phase I clinical trials. Protein. Expr. Purif. 2004, 33, 123–133.
- Frankel, A.E.; Weir, M.A.; Hall, P.D.; Hogge, D.E.; Rizzieri, D.A. Diptheria toxin-interleukin 3 fusion protein therapy of patients with elderly or relapsed/refractory acute myeloid leukemia (AML). J. Clin. Oncol. 2006, 24 (Suppl. S18), 6569.
- Frankel, A.E.; Weir, M.A.; Hall, P.D.; Holguin, M.; Cable, C.; Rizzieri, D.A.; Hogge, D.E. Induction of remission in patients with acute myeloid leukemia without prolonged myelosuppression using diphtheria toxin-interleukin 3 fusion protein. J. Clin. Oncol. 2007, 25 (Suppl. S18), 7068.
- Frankel, A.; Liu, J.-S.; Rizzieri, D.; Hogge, D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk. Lymphoma 2008, 49, 543–553.
