Epilepsy is a non-communicable disease of the brain that affects people of all ages. It is characterized by episodes of spontaneous and abnormal electrical activity in the brain. It is often accompanied by depression, anxiety, and substantially increased morbidity and mortality. A large number of third-generation antiepileptic drugs are available, but they have multiple side effects causing a decline in the quality of life. The inheritance and etiology of epilepsy are complex with multiple underlying genetic and epigenetic mechanisms. Different neurotransmitters play intricate functions to maintain the normal physiology of various neurons.
- anti-convulsants
- anti-epileptic drugs
- drug targets
- epileptogenesis
- non-communicable disease
- seizures
- transcriptional modifications
- pseudo-resistance
1. Introduction
2. Role of Genes, Genetics and Inheritance
The newly emerging genetic technology has played a significant role in the discovery of a variety of genes that are associated with epilepsy. The advancement of genomic techniques and gene sequencing has substantially enhanced the knowledge about the genetic variations taking place in the human genome. Studies estimate that there is an underlying genetic cause in about half of all cases of epilepsy [10]. Currently, with the emerging research of epigenetic biomarkers, MicroRNAs (miRNAs) have been assumed to play a significant role. MiRNA molecules 19-25 nucleotides long regulate gene expression as post-transcriptional modifiers [11]. Differential expression of more than 100 miRNAs has been reported in epilepsy. Among them, because of their predominant role in biological processes related to epilepsy, such as neurodegeneration, neuronal growth, and neuroinflammation, miR-132, miR-155, and miR-146a have been highlighted primarily [11]. Some cases of genetic mutations result in the core symptom of epilepsy, while changes in a few of the genes are responsible for malformations in the gross development of the brain that cause seizures. There are around 84 genes classified as epilepsy genes based on the OMIM database results. Mutation in these classified genes leads to epilepsy as a core symptom. There are approximately 73 genes that are categorized as neurodevelopment-associated epilepsy genes [10]. According to the OMIM database (https://www.ncbi.nlm.nih.gov/omim, accessed on 24 April 2021), there are about 536 genes responsible for causing associated diseases of epilepsy [10,13].
3. Epigenetics Involved in Epilepsy
Recent studies show epigenetics playing essential roles in temporal lobe epilepsy [17]. Therefore, studying the role of epigenetic changes in the development of the disease has become an emerging topic in the area of research. The knowledge of epigenetic mechanisms helps in providing the putative conceptual framework in the development of therapies that can help in the prevention of the disease. Epileptogenesis should be considered as a target point for developing therapy when there is an increment in the severity and frequency of impulsive recurrent seizures. Various processes that take place along with epileptogenesis are mossy fiber sprouting, dysfunction of adenosine together with gliosis, aberrant connectivity, neuronal cell loss, and neuroinflammation [18,19]. TCF4, MECP2, UBE3A, and CHD2 are some of the regulatory genes associated with epilepsy. Among these, the CHD2 gene is responsible for encoding a protein that remodels chromatin, and deregulation of CHD2 might have a downstream effect on other genes [20].
Epigenetic modifications are also responsible for many of the pathological changes that take place during epileptogenesis. Many changes have been shown to occur in the central nervous system cells that alter the gene expression due to DNA methylation and histone acetylation and methylation [12,21].
Various processes like histone modifications that involve either adding up or eliminating the acetyl or methyl groups are suggested to be associated with epileptogenesis. According to the hypothesis of DNA methylation implicated in epileptogenesis, seizures can induce epigenetic modifications and can exaggerate the process of epileptogenesis. DNA hypermethylation, along with the amplified activity of DNA methylating enzymes has been implicated in the development of experimental and human epilepsy [22].
Histone modification is one of the epigenetic mechanisms that have the considerable potential to alter the neuronal expression of genes by exerting their additive effects in a correlated manner [17]. The principal role of histone proteins is to support the tertiary and quaternary structure of the DNA. Disruption in these vital epigenetic machinery leads to various disorders such as epilepsy, autism, Rett syndrome, etc.
DNA methylation is dependent on several biochemical enzymatic reactions. One such reaction pathway is the S-adenosylmethionine-dependent transmethylation pathway, which is controlled by glycine and adenosine under the regulation of adenosine kinase (ADK). In chronic epilepsy, it is observed that there is an increase in the ADK and a resulting decline in adenosine, which leads to elevated DNA methylation in the brain. Thus, interference with methylation of DNA gives the new conceptual prospect to control and prevent epilepsy. Glycine modifying therapies can also be regarded as an alternative opportunity that affects the process of DNA methylation and, ultimately, the process of epileptogenesis. Therefore, understanding the epigenetics of epileptogenesis might help in the discovery and development of therapeutic interventions [18,26].
4. Neurotransmitter Release Machinery in Epilepsy
4.1. Glutamate Receptors
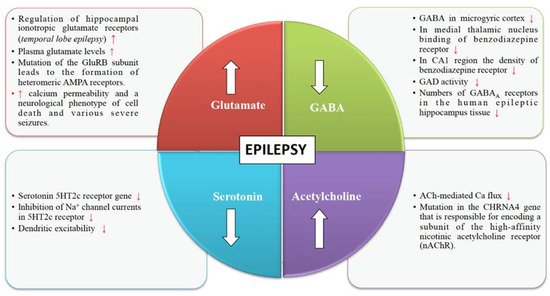
Glutamate is an excitatory neurotransmitter responsible for stimulating an increase in calcium and sodium conduction through ligand-gated ion channels (Figure 1). A wide spectrum of anti-convulsant properties is displayed by AMPA antagonists and NMDA antagonists in animal models with acute and chronic epilepsy [29]. Once seizures begin, the activity-dependent plasticity of the glutamate receptors becomes a vital feature of the epileptic brain.
4.2. GABA Receptors
Various pieces of evidence from several clinical and experimental data underline the role of GABA in the mechanism and management of epilepsy (Figure 1). The synaptic inhibition by GABA plays an essential role in regulating neuronal excitability, which has been linked to epilepsy. GABAergic neurotransmission that is controlled by Cl– permeable GABAA receptors can exhibit both seizure-repressing and -stimulating activity [33].
4.3. Cholinergic Receptors
The structural and functional diversity of the neuronal nicotinic acetylcholine receptors (nAChRs) perform modulatory functions throughout the mammalian brain (Figure 1). Nicotinic receptors are involved in various developmental mechanisms such as memory, attention, and learning. Disruptions in cholinergic mechanisms can lead to several disorders such as epilepsy, Parkinson’s disease, dementia, schizophrenia, autism, Alzheimer’s disease, etc. [37].
4.4. Serotonin Receptors
Serotonergic neurotransmission has been shown to have a potential role in epilepsy [42]. Serotonin receptors (5-HTRs) have been therefore considered as promising candidate targets for the development of new AEDs (Figure 1). Studies show 5-HT regulates a wide variety of focal and generalized seizures. Agents like 5-hydroxytryptophan and 5-HT reuptake blockers are known to increase the extracellular serotonin levels and hence inhibit focal as well as generalized seizures.
5. Drug Resistant Epilepsy
Drug-resistant epilepsy (DRE) is also known as refractory epilepsy or pharmaco-resistant epilepsy. It can be defined as a failure of two or more sufficient trials of tolerated, chosen, and appropriately used AEDs regimens, which can be administered as monotherapies or in combination to get relief from seizures. Around one out of four patients with seizures develop DRE [47]. DRE patients have increased risks of injuries, psychosocial problems, and premature death [48,49,50].
5.1. Alterations in the Drug Targets
5.2. The Inability of the Drugs to Reach Their Targets
5.3. Real Targets Missed by the Drugs
6. Non-Conventional Therapeutic Strategies
6.1. Ruling Out Pseudo-Resistance
6.2. Combination Therapy
6.3. Non-Drug Treatment
7. Modern Approaches for Treatment
7.1. MTOR Pathway
7.2. Inflammatory Pathways
7.3. Breakdown of Blood-Brain Barrier
| S. No. | Treatment Approaches | Interventions Used | Action Mechanism | Main Uses | References |
|---|---|---|---|---|---|
| A. | PHARMACEUTICAL APPROACHES | Gabapentin | Ca2+ blockage | Used for generalised and focal seizures. | [74,75] |
| (Anti-epileptic drugs) | Carbamazepine | Na+ channel blockage | Decrease nerve impulses that are responsible for causing seizures. | [74] | |
| Lamotrigine | Na+ channel blockage | Used as a first- line drug for generalized and focal seizures. | [60,75] | ||
| Tiagabine | GABA potentiation | Used for partial seizures in adjunctive therapy. | [74] | ||
| Zonisamide | Na+ channel blockage | Used for generalized and focal seizures. | [74,75] | ||
| Vigabatrin | GABA potentiation | Used for infantile spasms and for focal onset of seizures. | [74] | ||
| Perampanel | Glutamate (AMPA) antagonist | Used for partial seizures with focal onset. | [74,75] | ||
| B. | THERAPEUTIC APPROACHES | Progressive muscle relaxation | Tense a group of muscles while breathing in and relaxes them while breathing out. | Improves sleep and overall well-being. Enhances control over epilepsy by the patients. | [76] |
| Yoga | Release tension in key joints through combination of body postures. | Decrease in automatic dysfunction during onset of seizures. | [76,77] | ||
| Cognitive behavioural therapy | Restructuring of maladaptive thought patterns. | Improvement in anxiety and depression and enhanced psychosocial functioning. | [76,78] | ||
| Vagus nerve stimulation | Used to generate impulse through electric current in vagus nerve. | Used as an adjunctive therapy for partial onset of seizures. | [65] | ||
| C. | NATURAL APPROACHES | Ketogenic diet | Neurotransmitter modulation in brain by ketone bodies. | Successful in reducing seizures and enhancing motor function. | [27,64,79] |
| Vitamin D3 | Increase Ca2+ uptake and decrease neuronal excitability. | Produces anti-convulsant effect and prevent seizures. | [80,81] | ||
| Herbal treatments | Found to be involved in potentiation of GABAergic activity in brain. | Herbal medications control epileptic seizures and reduce side effects and increase cognitive effects of AEDs. | [82,83] |
8. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050470
