Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Marine natural compounds suppress or kill plant pathogenic pathogens through different mechanisms, including affecting microbial cell wall synthesis, cell membrane permeability, fatty acid metabolism, respiratory system, cytoskeleton, bacterial quorum sensing (QS), as well as inducing plant immune system for inhibition.
- marine natural products
- plant pathogens
- bioactive substances
- chemical control
- antimicrobial mechanism
1. Affect Cell Wall Structures
Cell wall is the outermost structure of the plant pathogenic fungi and bacteria, which plays important role in maintaining cell shape and integrity. It also maintains normal metabolism, ion exchange, and osmotic pressure in cells [1]. Some marine compounds can inhibit the formation of microbial cell walls, thus suppress the growth or kill the pathogens. For instance, Chakraborty et al. isolated compounds 13–17 from a marine bacterium B. subtilis 109GGC020. Compounds 13–17 inhibit cell wall synthesis of M. oryzae to inhibit its growth [2]. Marine natural products can destroy structure of the fungal cell wall component glucosamine to inhibit the cell wall synthesis and growth. For example, microalgal phenolic extracts (MPE) were isolated from marine microalgae Nannochloropsis sp. and Spirulina sp., which can destroy the glucosamine structure of F. graminearum CQ244 and reduce glucosamine production by 15% [3]. Chitin is another fungal cell wall key component, which can be degraded by chitinase [4]. A chitinase was identified from the marine bacterium B. pumilus JUBCH08, which was proved to degrade the cell wall of F. oxysporum and inhibit its growth [5].
2. Affect Cell Membrane Permeability
The cell membrane is a lipid bilayer semi-permeable membrane, which controls the two-way flow of substances inside and outside the cell of the plant pathogens. The microbial cell membrane is another most common target of marine natural compounds. For example, antifungal ethyl acetate extract from marine-derived Streptomyces sp. AMA49 can destroy the cell membrane of M. oryzae to suppress fungal growth [6]. A series of other marine-derived natural compounds can also achieve their antibacterial effects by destroying the cell membrane of different plant pathogens [7][8][9][10][11][12]. Compound 9 isolated from B. velezensis 11-5 can selectively bind with phospholipids in the cell membrane of M. oryzae, thus affecting the membrane structure [13]. The microalgal phenolic extracts, which are isolated from Nanochoropsis and Spirulina, can combine with ergosterol on the membrane (MPE) of F. graminearum cells to elevate plasma membrane permeability and lead to the leakage of proteins, nucleotides, amino acids, sugars, and salts, therefore leading to the death of fungi [14].
3. Affect Fatty Acid Metabolism
Fatty acid metabolism is very important for the functional appressorium formation in some plant pathogenic fungi. When the fatty acid metabolism of fungi is blocked, the fungi cannot penetrate plant cells [15][16][17]. Interestingly, it is reported that halisulfate 1 and bromophenols, the marine-derived metabolites, could inhibit the activity of M. oryzae isocitrate lyase, thus inhibiting the fatty acid metabolism, which will affect mature appressorium formation and penetration of M. oryzae [18][19].
4. Affect Respiratory System
Marine natural compounds can also target the respiratory electron transport system of plant pathogens to inhibit their respiration, through which they can inhibit the growth of pathogens. For example, compound 26 (Figure 1) isolated from marine Myxobacterium can breaking the electron flow by targeting the cytochrome b-c1 segment [20], and therefore inhibits the growth of plant pathogens.
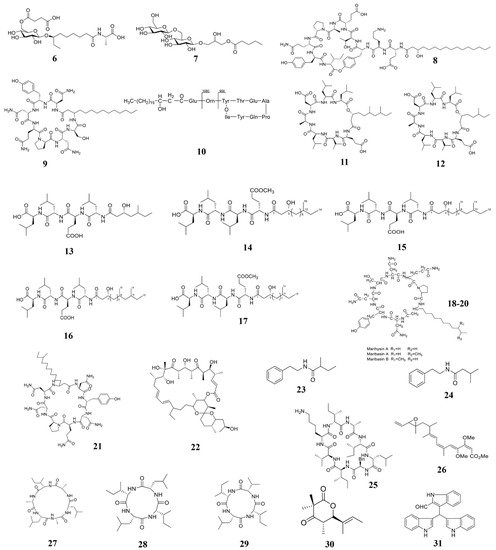
Figure 1. Chemical structures of the compounds identified from marine bacteria. 6 Ieodoglucomide C, 7 ieodoglycolipid, 8 plipastatin A1, 9 iturin A, 10 Fengycin BS155, 11–12 gageopeptins A and B, 13–16 gageopeptides A–D, 17 gageotetrin B, 18–20 Lipopeptides, 21 mojavensin A, 22 oligomycin A, 23 2-methyl-N-(2′-phenylethyl)-butanamide, 24 3-methyl-N-(2′-phenylethyl)-butanamide, 25 Champacyclin, 26 Haliangicin, 27–29 Halolitoralin, 30 helicascolide C, 31 Trisindolal.
5. Affect Cytoskeleton Formation
The cytoskeleton also plays important roles for fungal development and infection processes [21], whose components could be bound by the marine compounds and inhibit fungal growth and infection. For instance, the marine organisms derived compounds 46–48 (Figure 2) can inhibit the growth of Colletotrichum species and B. cinerea [22], the mechanism is that compounds 46–48 target the fungal β-tubulin proteins and subsequently suppress cell division [22].
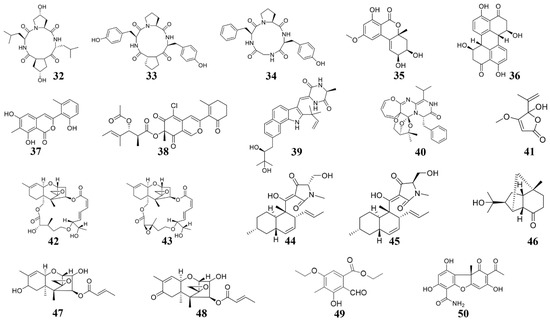
Figure 2. Chemical structures of the compounds identified from marine fungi. 32 Cyclo-(L-leucyl-trans-4-hydroxy-L-prolyl-D-leucyl-trans-4-hydroxy-L-proline), 33 cyclo (D-Pro-L-Tyr-L-Pro-L-Tyr), 34 cyclo (Gly-L-Phe-L-Pro-L-Tyr), 35 Benzopyranone, 36 Stemphyperylenol, 37 pleosporalone A, 38 pleosporalones B, 39 rubrumazine B, 40 Varioxepine A, 41 Penicillic acid, 42 roridin A, 43 roridin D, 44 equisetin, 45 epi-equisetin, 46–48 sesquiterpenes, 49 ethyl 5-ethoxy-2-formyl-3-hydroxy-4-methylbenzoate, 50 (-)-cercosporamide.
6. Affect Bacterial QS System
QS system can help bacteria to monitor the quantity change of itself or other bacteria in the surrounding environment, according to the concentration change of specific signal molecule autoinducer. When the signal molecule reaches a certain concentration threshold, it can start the expression of related genes in the bacteria to adapt to the environmental changes. When the QS system of bacteria is blocked, bacteria cannot communicate with the surrounding environment and failed to infect the host plant. Interestingly, some marine natural compounds can also target and interrupt the bacterial QS system to prevent infection of the bacterial pathogens [23][24]. For example, 2-methyl-n-(2’-phenylethyl)-butanamide and 3-methyl-n-(2’-phenylethyl)-butanamide, two marine-derived compounds, can destroy the QS system of rice pathogenic bacterium B. glumae (ATCC 333,617), therefore interrupt virulence feature production, including proteases, toxins, as well as some other immune-evasion factors [25]. In this situation, the QS signal is blocked by the marine compounds, and the bacteria fail to attack the host.
7. Induction of the Plant Immune System
Some marine natural products can not directly inhibit or kill bacteria against plant pathogens, but be used as elicitors to stimulate the plant immune system to inhibit or kill bacteria, which serve as an indirect antibacterial or bactericidal effect [26]. Ji et al. and Righini et al. isolated a series of compounds (60, 61, polysaccharides) from marine organisms (Figure 3) [27][28]. The activity test of these compounds showed that they could not directly inhibit the growth of plant pathogens, but stimulate the plants to resist to plant pathogens [27][28]. This elegant work clearly showed that the marine compounds could indirectly inhibit plant pathogens by inducing the host immune system.
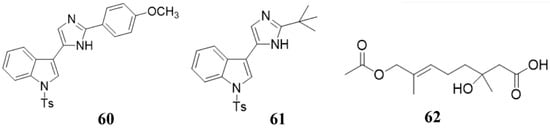
Figure 3. Chemical structures of the compounds 60 (nortopsentin alkaloid 2e), 61 (nortopsentin alkaloid 2k), and 62 penicimonoterpene (±).
This entry is adapted from the peer-reviewed paper 10.3390/md19020069
References
- Dorr, T. Understanding tolerance to cell wall-active antibiotics. Ann. N. Y. Acad. Sci. 2020.
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae triticum. Front. Microbiol. 2020, 11, 665.
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. As a source of antifungal compounds to mitigate contamination by Fusarium graminearum species complex. Curr. Microbiol. 2019, 76, 930–938.
- Lozoya-Perez, N.E.; Clavijo-Giraldo, D.M.; Martinez-Duncker, I.; Garcia-Carnero, L.C.; Lopez-Ramirez, L.A.; Nino-Vega, G.A.; Mora-Montes, H.M. Influences of the culturing media in the virulence and cell wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. J. Fungi (Basel) 2020, 6, 323.
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317–322.
- Nair, V.; Schuhmann, I.; Anke, H.; Kelter, G.; Fiebig, H.H.; Helmke, E.; Laatsch, H. Marine bacteria, XLVII—Psychrotolerant bacteria from extreme antarctic habitats as producers of rare bis- and trisindole alkaloids. Planta Med. 2016, 82, 910–918.
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84.
- Zhao, D.L.; Wang, D.; Tian, X.Y.; Cao, F.; Li, Y.Q.; Zhang, C.S. Anti-phytopathogenic and cytotoxic activities of crude extracts and secondary metabolites of marine-derived fungi. Mar. Drugs 2018, 16, 36.
- Zhao, D.; Han, X.; Wang, D.; Liu, M.; Gou, J.; Peng, Y.; Liu, J.; Li, Y.; Cao, F.; Zhang, C. Bioactive 3-decalinoyltetramic acids derivatives from a marine-derived strain of the fungus Fusarium equiseti D39. Front. Microbiol. 2019, 10, 1285.
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851.
- Lopez-Abarrategui, C.; Alba, A.; Silva, O.N.; Reyes-Acosta, O.; Vasconcelos, I.M.; Oliveira, J.T.; Migliolo, L.; Costa, M.P.; Costa, C.R.; Silva, M.R.; et al. Functional characterization of a synthetic hydrophilic antifungal peptide derived from the marine snail Cenchritis muricatus. Biochimie 2012, 94, 968–974.
- Wang, K.J.; Cai, J.J.; Cai, L.; Qu, H.D.; Yang, M.; Zhang, M. Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides 2009, 30, 638–646.
- Ma, Z.; Zhang, S.; Sun, K.; Hu, J. Identification and characterization of a cyclic lipopeptide iturin A from a marine-derived Bacillus velezensis 11-5 as a fungicidal agent to Magnaporthe oryzae in rice. J. Plant Dis. Protect. 2019, 127, 15–24.
- Scaglioni, P.T.; Scarpino, V.; Marinaccio, F.; Vanara, F.; Furlong, E.B.; Blandino, M. Impact of microalgal phenolic extracts on the control of Fusarium graminearum and deoxynivalenol contamination in wheat. World Mycotoxin J. 2019, 12, 367–378.
- Lanver, D.; Mendoza-Mendoza, A.; Brachmann, A.; Kahmann, R. Sho1 and Msb2-related proteins regulate appressorium development in the smut fungus Ustilago maydis. Plant Cell 2010, 22, 2085–2101.
- bin Yusof, M.T.; Kershaw, M.J.; Soanes, D.M.; Talbot, N.J. FAR1 and FAR2 regulate the expression of genes associated with lipid metabolism in the rice blast fungus Magnaporthe oryzae. PLoS ONE 2014, 9, e99760.
- Deng, S.; Gu, Z.; Yang, N.; Li, L.; Yue, X.; Que, Y.; Sun, G.; Wang, Z.; Wang, J. Identification and characterization of the peroxin 1 gene MoPEX1 required for infection-related morphogenesis and pathogenicity in Magnaporthe oryzae. Sci. Rep. 2016, 6, 36292.
- Shin, D.S.; Lee, T.H.; Lee, H.S.; Shin, J.; Oh, K.B. Inhibition of infection of the rice blast fungus by halisulfate 1, an isocitrate lyase inhibitor. FEMS Microbiol. Lett. 2007, 272, 43–47.
- Oh, K.-B.; Lee, J.H.; Chung, S.-C.; Shin, J.; Shin, H.J.; Kim, H.-K.; Lee, H.-S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 104–108.
- Pesic, A.; Baumann, H.I.; Kleinschmidt, K.; Ensle, P.; Wiese, J.; Süssmuth, R.D.; Imhoff, J.F. Champacyclin, a new cyclic octapeptide from Streptomyces strain C42 isolated from the Baltic Sea. Mar. Drugs 2013, 11, 4834–4857.
- Lian, N.; Wang, X.; Jing, Y.; Lin, J. Regulation of cytoskeleton-associated protein activities: Linking cellular signals to plant cytoskeletal function. J. Integr. Plant Biol. 2020.
- Du, F.Y.; Ju, G.L.; Xiao, L.; Zhou, Y.M.; Wu, X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs 2020, 18, 165.
- Haque, M.; Islam, S.; Sheikh, M.A.; Dhingra, S.; Uwambaye, P.; Labricciosa, F.M.; Iskandar, K.; Charan, J.; Abukabda, A.B.; Jahan, D. Quorum sensing: A new prospect for the management of antimicrobial-resistant infectious diseases. Expert. Rev. Anti. Infect. Ther. 2020, 1–16.
- Soto-Aceves, M.P.; Cocotl-Yanez, M.; Servin-Gonzalez, L.; Soberon-Chavez, G. The Rhl quorum sensing system is at the top of the regulatory hierarchy under phosphate limiting conditions in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2020.
- Betancur, L.A.; Forero, A.M.; Vinchira-Villarraga, D.M.; Cardenas, J.D.; Romero-Otero, A.; Chagas, F.O.; Pupo, M.T.; Castellanos, L.; Ramos, F.A. NMR-based metabolic profiling to follow the production of anti-phytopathogenic compounds in the culture of the marine strain Streptomyces sp. PNM-9. Microbiol. Res. 2020, 239, 126507.
- de Almeida, C.L.; Falcao Hde, S.; Lima, G.R.; Montenegro Cde, A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza Mde, F.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573.
- Righini, H.; Baraldi, E.; Garcia Fernandez, Y.; Martel Quintana, A.; Roberti, R. Different antifungal activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299.
- Ji, X.; Guo, J.; Liu, Y.; Lu, A.; Wang, Z.; Li, Y.; Yang, S.; Wang, Q. Marine-natural-product development: First discovery of nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J. Agric. Food Chem. 2018, 66, 4062–4072.
This entry is offline, you can click here to edit this entry!
