Steroids are a pivotal class of hormones with a key role in growth modulation and signal transduction in multicellular organisms. Synthetic steroids are widely used to cure large array of viral, fungal, bacterial, and cancerous infections. Brassinosteroids (BRs) are a natural collection of phytosterols, which have structural similarity with animal steroids. BRs are dispersed universally throughout the plant kingdom. These plant steroids are well known to modulate a plethora of physiological responses in plants leading to improvement in quality as well as yield of food crops. Moreover, they have been found to play imperative role in stress-fortification against various stresses in plants. Over a decade, BRs have conquered worldwide interest due to their diverse biological activities in animal systems.
- brassinosteroids
- anticancerous
- antiviral
- antibacterial
- ecdysteroidal activities
1. Introduction
2. Therapeutic Role of BRs
2.1. Anticancerous/Antiproliferative Activities
2.2. Antiviral Activities
| Viruses | (% Inhibition) | |
|---|---|---|
| 28-homocastaterone | Brassinolide | |
| Poliovirus type 1 (RNA virus) | 85 | 96 |
| Vesicular stomatitis Virus Indiana strain (RNA virus) | 23 | 100 |
| Herpes simplex virus-1 F strain (tk+) (DNA virus) | 50 | 96 |
| Herpes simplex virus-1 B2006 strain (tk−) (DNA virus) | 35 | 100 |
| Herpes simplex virus-1 G strain (tk+) (DNA virus) | 48 | 98 |
| Junin virus IV 4454 strain (RNA virus) | 79 | 74 |
| Tacaribe virus TRLV 11573 strain (RNA virus) | 99 | 55 |
| Pichinde virus AN3739 strain (RNA virus) | 98 | 67 |
| Measles virus Brasil/001/91 (RNA virus) | 50 | 100 |
2.3. Antiherpetic Activities
2.4. Antifungal and Antibacterial Activities
2.5. Anti-Inflammatory Activities
2.6. Antiangiogenic Activities and Antigenotoxic
2.7. Anticholesteromic Action
2.8. Ecdysteroidal Activities
2.9. Anabolic Activities
This entry is adapted from the peer-reviewed paper 10.3390/biom10040572
References
- Harborne, J.B. Flavonoid profiles in the compositae. In The Biology and Chemistry of the Compositae; Heywood, V.H., Harborne, J.B., Turner, B.L., Eds.; Academic Press: London, UK, 1977; pp. 359–384.
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93.
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79.
- Tarkowská, D. Plants are Capable of Synthesizing Animal Steroid Hormones. Molecules 2019, 24, 2585.
- Czerpak, R.; Szamrej, I.K. The effect of β-estradiol and corticosteroids on chlorophylls and carotenoids content in Wolffia arrhiza(L.) Wimm. (Lemnaceae) growing in municipal bialystok tap water. Pol. J. Environ. Stud. 2003, 12, 677–684.
- Szamrej, I.K.; Czerpak, R. The effect of sex steroids and corticosteroids on the content of soluble proteins, nucleic acids and reducing sugars in Wolffia arrhiza(L.) Wimm. (Lemnaceae). Pol. J. Environ. Stud. 2004, 13, 565–571.
- Shpakovski, G.V.; Spivak, S.G.; Berdichevets, I.N.; Babak, O.G.; Kubrak, S.V.; Kilchevsky, A.V.; Aralov, A.V.; Slovokhotov, I.Y.; Shpakovski, D.G.; Baranova, E.N.; et al. A key enzyme of animal steroidogenesis can function in plants enhancing their immunity and accelerating the processes of growth and development. BMC Plant Biol. 2017, 17, 189.
- Caspi, E.; Lewis, D.O.; Platak, D.M.; Thimann, K.V.; Winter, A. Biosynthesis of plant sterols. Conversion of cholesterol to pregnenolone in Digitalis purpurea. Experientia 1966, 22, 506–507.
- Caspi, E.; Lewis, D.O. Progesterone: Its possible role in the biosynthesis of cardenolides in Digitalis lanata. Science 1967, 156, 519–520.
- Sonawane, P.D.; Heinig, U.; Panda, S.; Gilboa, N.S.; Yona, M.; Kumar, S.P.; Alkan, N.; Unger, T.; Bocobza, S.; Pliner, M.; et al. Short-chain dehydrogenase/reductase governs steroidal specialized metabolites structural diversity and toxicity in the genus Solanum. Proc. Natl. Acad. Sci. 2018, 115, E5419–E5428.
- Bennett, R.D.; Heftmann, E. Biosynthesis of pregnenolone from cholesterol in Haplopappusheterophyllus. Phytochemistry 1966, 5, 747–754.
- Bennett, R.; Heftmann, E.; Winter, B. Conversion of sitosterol to progesterone by Digitalis Lanata. Naturwissenschaften 1969, 56, 463.
- Caspi, E.; Hornby, G.M. Biosynthesis of plant sterols-III. Mechanism of saturation on ring B in pregnenolone during its conversion to digitoxigenin in Digitalis lanata. Phytochemistry 1968, 7, 423–427.
- Bennett, R.D.; Heftmann, E.; Joly, R.A. Biosynthesis of diosgenin from 26 hydroxy cholesterol in dioscorea. Phytochemistry 1970, 9, 349–353.
- Heftmann, E. Functions of sterols in plants. Lipids 1971, 6, 128–133.
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Carter Cook, J., Jr. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217.
- Yokota, T.; Arima, M.; Takahashi, N. Castasterone, a new phytosterol with planthormone potency from chestnut insect gall. Tetrahedron Lett. 1982, 23, 1275–1278.
- Bhardwaj, R.; Arora, H.K.; Nagar, P.K.; Thukral, A.K. Brassinosteroids-A novel group of plant hormones. In Plant Molecular Physiology-Current Scenario and Future Projections; Trivedi, P.C., Ed.; Aaviskar Publisher: Jaipur, India, 2006; pp. 58–84.
- Bhardwaj, R.; Kaur, S.; Nagar, P.K.; Arora, H.K. Isolation and characterization of brassinosteroids from immature seeds of Camellia sinensis(O) Kuntze. Plant Growth Regul. 2007, 53, 1–5.
- Clouse, S.D.; Sasse, J.M. Brassinosteriods: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451.
- Pereira-Netto, A.B.; Schaefer, S.; Galagovsky, L.R.; Ramirez, J.A. Brassinosteroid-driven modulation of stem elongation and apical dominance: Applications in micropropagation. In Brassinosteroids; Springer: Dordrecht, The Netherlands, 2003; pp. 129–157.
- Nakamura, A.; Higuchi, K.; Goda, H.; Fujiwara, M.T.; Sawa, S.; Koshiba, T.; Shimada, Y.; Yoshida, S. Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol. 2003, 133, 1843–1853.
- Khripach, V.A.; Zhabinskii, V.N.; de Groot, A. Twenty years of Brassinosteriods: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447.
- Clouse, S.D. Brassinosteroids. Arab. Book Am. Soc. Plant Biol. 2011, 9.
- Krishna, P. Brassinosteroid-Mediated Stress Responses. J. Plant Growth Regul. 2003, 22, 289–297.
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173.
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26.
- Kohli, S.K.; Bali, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Sharma, A.; Verma, V.; Ohri, P.; Mir, B.A.; Kaur, R.; et al. A Current Scenario on Role of Brassinosteroids in Plant Defense Triggered in Response to Biotic Challenges. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 367–388.
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185.
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2010, 60, 406–416.
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151.
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J. Plant Growth Regul. 2015, 34, 509–518.
- Swaczynova, J.; Sisa, M.; Hnilickova, J.; Kohout, L.; Strnad, M. Synthesis, biological, immunological and anticancer properties of a new brassinosteroid ligand. Pol. J. Chem. 2006, 80, 629–636.
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46.
- Peres, A.L.G.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331.
- Malíkova, J.; Swaczynova, J.; Kolar, Z.; Strnad, M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 2008, 69, 418–426.
- Oklestkova, J.; Hoffmannova, L.; Steigerova, J.; Kohout, L.; Kolar, Z.; Strnad, M. Natural Brassinosteroids for Use for Treating Hyperproliferation, Treating Proliferative Diseases and Reducing Adverse Effects of Steroid Dysfunction in Mammals, Pharmaceutical Composition and Its Use. U.S. Patent No. 20100204460, 20 August 2008.
- Obakan, P.; Barrero, C.; Coker-Gurkan, A.; Arisan, E.D.; Merali, S.; Palavan-Unsal, N. SILAC-based mass spectrometry analysis reveals that epibrassinolide induces apoptosis via activating endoplasmic reticulum stress in prostate cancer cells. PLoS ONE 2015, 10, e0135788.
- Esposito, D.; Rathinasabapathy, T.; Schmidt, B.; Shakarjian, M.P.; Komarnytsky, S.; Raskin, I. Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair Regen. 2013, 21, 688–696.
- Wachsman, M.B.; Ramírez, J.A.; Talarico, L.B.; Galagovsky, L.R.; Coto, C.E. Antiviral activity of natural and synthetic brassinosteroids. Curr. Med. Chem. Anti-Infect. Agents 2004, 3, 163–179.
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288.
- Michelini, F.M.; Zorrilla, P.; Robello, C.; Alche, L.E. Immunomodulatory activity of an anti-HSV-1 synthetic stigmastaneanalog. Bioorganic Med. Chem. 2013, 21, 560–568.
- Mehtiev, A.R.; Misharin, A.Y. Biological activity of phytosterols and their derivatives. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2008, 2, 1–17.
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270.
- Greenwell, M.; Rahman, P.K.S.M. Medicinal plants: their use in anticancer treatment. Int. J. Pharma. Sci. Res. 2015, 6, 4103.
- Kaushik, P.; Pahwa, P.; Kaushik, P.P. A Comprehensive Review on Medicinal Plants with Anticancer Activity. Global J. Pharma Edu. Res. 2018, 3.
- Zhabinskii, V.N.; Khripach, N.B.; Khripach, V.A. Steroid plant hormones: effects outside plant kingdom. Steroids 2015, 97, 87–97.
- Ramirez, J.A.; Michelini, F.M.; Galagovsky, L.R.; Berra, A.; Alche, L.E. Antiangiogenic Brassinosteroid Compounds. U.S. Patent 2013088400, 17 November 2015.
- Denmeade, S.R.; Lin, X.S.; Isaacs, J.T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996, 28, 251–265.
- Weisburger, J.H. Worldwide prevention of cancer and other chronic diseases based on knowledge of mechanisms. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 331–337.
- Parl, F.F. Estrogens, Estrogen Receptor and Breast Cancer; IOS Press: Amsterdam, The Netherlands, 2000.
- Franek, F.; Eckschlager, T.; Kohout, L. 24-Epibrassinolide at subnanomolar concentrations modulates growth and production characteristics of a mouse hybridoma. Collect. Czechoslov. Chem. Commun. 2003, 68, 2190–2200.
- Steigerova, J.; Oklestkova, J.; Levkova, M.; Rarova, L.; Kolar, Z.; Strnad, M. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Interactions 2010, 188, 487–496.
- Steigerova, J.; Rarova, L.; Oklestkova, J.; Krizova, K.; Levkova, M.; Svachova, M.; Kolar, Z.; Strnad, M. Mechanisms of natural brassinosteroid-induced apoptosis of prostate cancer cells. Food Chem. Toxicol. 2012, 50, 4068–4076.
- Obakan, P.; Arisan, E.D.; Calcabrini, A.; Agostinelli, E.; Bolkent, S.; Palavan-Unsal, N. Activation of polyamine catabolic enzymes involved in diverse responses against epibrassinolide-induced apoptosis in LNCaP and DU145 prostate cancer cell lines. Amino Acids 2014, 46, 553–564.
- Wu, Y.D.; Lou, Y.J. Brassinolide, a plant sterol from pollen of Brassica napus L., induces apoptosis in human prostate cancer PC-3 cells. Pharmazie 2007, 62, 392–395.
- Coskun, D.; Obakan, P.; Arisan, E.D.; Çoker-Gürkan, A.; Palavan-Ünsal, N. Epibrassinolide alters PI3K/MAPK signaling axis via activating Foxo3a-induced mitochondria-mediated apoptosis in colon cancer cells. Exp. Cell Res. 2015, 338, 10–21.
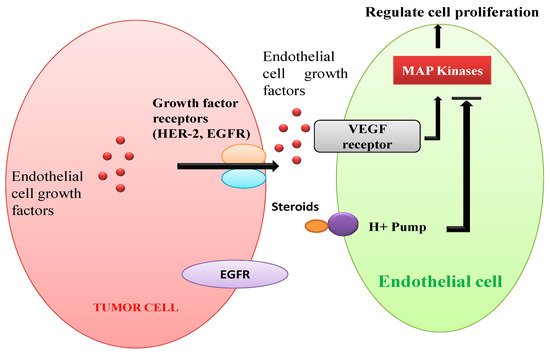
- Rarova, L.; Zahler, S.; Liebl, J.; Krystof, V.; Sedlak, D.; Bartunek, P.; Kohout, L.; Strnad, M. Brassinosteroids inhibit in vitro angiogenesis in human endothelial cells. Steroids 2012, 77, 1502–1509.
- Rárová, L.; Sedlák, D.; Oklestkova, J.; Steigerová, J.; Liebl, J.; Zahler, S.; Bartůněk, P.; Kolář, Z.; Kohout, L.; Kvasnica, M.; et al. The novel brassinosteroid analog BR4848 inhibits angiogenesis in human endothelial cells and induces apoptosis in human cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2018, 178, 263–271.
- Patil, M.R.; Elbert, T.; Keri, R.S. Labelling of brassinosteroids by isotopes of hydrogen and carbon. RSC Adv. 2015, 5, 39726–39745.
- Calil, I.P.; Fontes, E.P. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2016, 119, 711–723.
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272.
- Liebrand, T.W.H.; van den Burg, H.A.; Joosten, M.H.A.J. Two for all: Receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014, 19, 123–132.
- Kørner, C.J.; Klauser, D.; Niehl, A.; Domínguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant Microbe Interact. 2013, 26, 1271–1280.
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898.
- Yang, H.; Gou, X.; He, K.; Xi, D.; Du, J.; Lin, H.; Li, J. BAK1 and BKK1 in Arabidopsis thaliana confer reduced susceptibility to turnip crinkle virus. Eur. J. Plant Pathol. 2010, 127, 149–156.
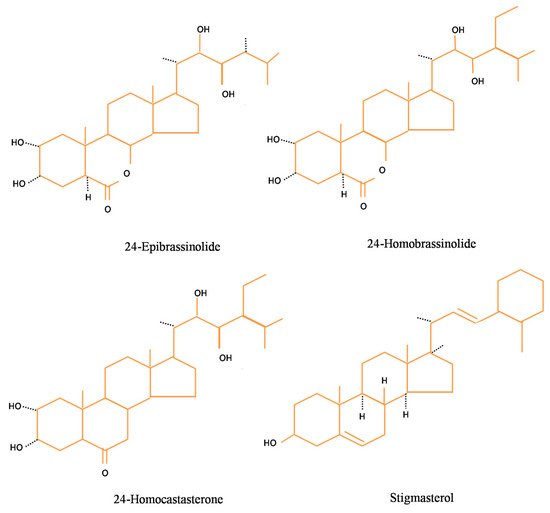
- Zhang, D.W.; Deng, X.G.; Fu, F.Q.; Lin, H.H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 2015, 241, 875–885.
- Deng, X.G.; Zhu, T.; Peng, X.J.; Xi, D.H.; Guo, H.; Yin, Y.; Zhang, D.W.; Lin, H.H. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 2016, 6, 20579.
- Wachsman, M.B.; Castilla, V. Antiviral properties of brassinosteroids. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 57–71.
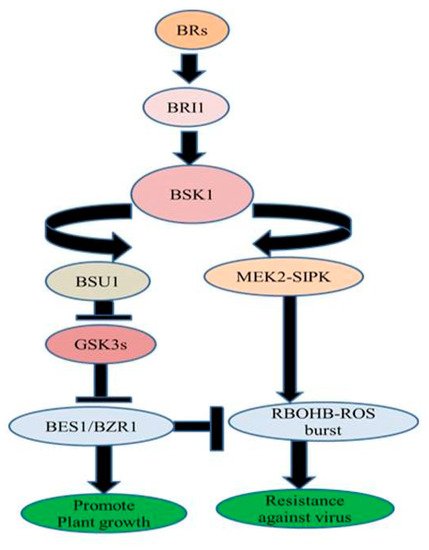
- Wachsman, M.B.; Ramirez, J.A.; Galagovsky, L.R.; Coto, C.E. Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivir. Chem. Chemother. 2002, 13, 61–66.
- Romanutti, C.; Castilla, V.; Coto, C.E.; Wachsman, M.B. Antiviral effect of a synthetic brassinosteroid on the replication of vesicular stomatitis virus in Vero cells. Int. J. Antimicrob. Agents 2007, 29, 311–316.
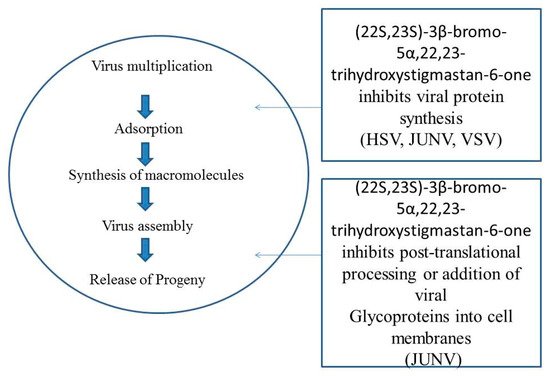
- Castilla, V.; Larzabal, M.; Sgalippa, N.A.; Wachsman, M.B.; Coto, C.E. Antiviral mode of action of a synthetic brassinosteroid against Junin virus replication. Antivir. Res. 2005, 68, 88–95.
- Zou, L.J.; Deng, X.G.; Zhang, L.E.; Zhu, T.; Tan, W.R.; Muhammad, A.; Zhu, L.J.; Zhang, C.; Zhang, D.W.; Lin, H.H. Nitric oxide as a signaling molecule in brassinosteroid-mediated virus resistance to Cucumber mosaic virus in Arabidopsis thaliana. Physiol. Plant. 2018, 163, 196–210.
- Petrera, E.; Níttolo, A.G.; Alche, L.E. Antiviral Action of Synthetic Stigmasterol Derivatives on Herpes Simplex Virus Replication in Nervous Cells In Vitro. BioMed Res. Int. 2014, 1–9.
- Wickham, S.; Carr, D.J. Molecular mimicry versus bystander activation: Herpetic stromal keratitis. Autoimmunity 2004, 37, 393–397.
- Toma, H.S.; Murina, A.T.; Areaux, R.G., Jr.; Neumann, D.M.; Bhattacharjee, P.S.; Foster, T.P.; Kaufman, H.E.; Hill, J.M. Ocular HSV-1 latency, reactivation and recurrent disease. Semin. Ophthalmol. 2008, 23, 249–273.
- Jiang, X.; Chentoufi, A.A.; Hsiang, C.; Carpenter, D.; Osorio, N.; BenMohamed, L.; Fraser, N.W.; Jones, C.; Wechsler, S.L. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J. Virol. 2011, 85, 2325–2332.
- Sabah, M.; Mulcahy, J.; Zeman, A. Herpes simplex encephalitis. Br. Med. J. 2012, 344, e3166.
- Kamei, S.; Sekizawa, T.; Shiota, H.; Mizutani, T.; Itoyama, Y.; Takasu, T.; Hirayanagi, K. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1544–1549.
- Kamei, S.; Taira, N.; Ishihara, M.; Sekizawa, T.; Morita, A.; Miki, K.; Itoyama, Y. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine 2009, 46, 187–193.
- Castilla, V.; Ramirez, J.; Coto, C.E. Plant and animal steroids a new hope to search for antiviral agents. Curr. Med. Chem. 2010, 17, 1858–1873.
- Ramirez, J.A.; Teme Centurion, O.M.; Gros, E.G.; Galagovsky, L.R. Synthesis and bioactivity evaluation of brassinosteroid analogs. Steroids 2000, 65, 329–337.
- Lan, W.; Petznick, A.; Heryati, S.; Rifada, M.; Tong, L. Nuclear Factor-κB: Central regulator in ocular surface inflammation and diseases. Ocul. Surf. 2012, 10, 137–148.
- Berra, A.; Michelini, F.M.; Ramirez, J.; Galagovsky, L.; Alche, L. In vitro and in vivo Anti-Herpetic and Anti-Inflammatory Activities of a New Synthetic Brassinosteroid Analogue. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5519.
- Michelini, F.M.; Ramirez, J.A.; Berrac, A.; Galagovsky, L.R.; Alche, L.E. Anti-herpetic and anti-inflammatory activities of two new synthetic 22,23-dihydroxylated stigmastane derivatives. J. Steroid Biochem. Mol. Biol. 2008, 111, 111–116.
- Van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull. World Heal. Organ. 1972, 46, 845–852.
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.Z.; Persidsky, Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J. Leukoc. Biol. 2013, 93, 801–810.
- Khripach, V.A.; Zhabinskii, V.N.; Konstantinova, O.V.; Khripach, N.B.; Antonchik, A.V.; Antonchik, A.P.; Schneider, B. Preparation of (25R)- and (25S)-26-functionalized steroids as tools for biosynthetic studies of cholic acids. Steroids 2005, 70, 551–562.
- Khripach, V.A.; Sviridov, O.V.; Litvinovskaya, R.P.; Pryadko, A.G.; Drach, S.V.; Zhabinskii, V.N. Analysis of brassinosteroids. Pol. J. Chem. 2006, 80, 651–654.
- Knickelbein, J.E.; Hendricks, R.L.; Charukamnoetkanok, P. Management of Herpes Simplex Virus Stromal Keratitis: An Evidence-based Review. Surv. Ophthalmol. 2009, 54, 226–234.
- Michelini, F.M.; Ramirez, J.A.; Berra, A.; Galagovsky, L.R.; Alche, L.E. In vitroand in vivoantiherpetic activity of three new synthetic brassinosteroid analogues. Steroids 2004, 69, 713–720.
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8.
- Pshenichnaya, L.A.; Khripach, V.A.; Volynetz, A.P.; Prokkhorchik, R.A.; Manzhelesova, N.E.; Morozik, G.V. Brassinosteroids and resistance of barley plants to leaf diseases. In Problems of Experimental Botany; Parfenov, V.I., Ed.; Byelorussian Science: Minsk, Belarus, 1997; pp. 210–217.
- Roth, U.; Friebe, A.; Schnabl, H. Resistance induction in plants by a brassinosteroid-containing extract of LychnisViscaria L. Z. Naturfor. J. Biosci. 2000, 55, 552–559.
- Bibi, N.; Ahmed, I.M.; Fan, K.; Dawood, M.; Li, F.; Yuan, S.; Wang, X. Role of brassinosteroids in alleviating toxin-induced stress of Verticillium dahliae on cotton callus growth. Environ. Sci. Pollut. Res. 2017, 24, 12281–12292.
- Bibi, N.; Fan, K.; Dawood, M.; Nawaz, G.; Yuan, S.; Xuede, W. Exogenous application of epibrassinolide attenuated Verticillium wilt in upland cotton by modulating the carbohydrates metabolism, plasma membrane ATPases and intracellular osmolytes. Plant Growth Regul. 2014, 73, 155.
- Zhua, Z.; Zhanga, Z.; Qina, G.; Tiana, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55.
- Ali, S.S.; Kumar, G.S.; Khan, M.; Doohan, F.M. Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 2013, 103, 1260–1267.
- Lin, L.J.; Tai, S.S.K.; Peng, C.C.; Tzen, J.T.C. Steroleosin, a sterol-binding dehydrogenase in seed oil bodies. Plant Physiol. 2002, 128, 1200–1211.
- Churikova, V.V.; Vladimirova, I.N. Effect of epibrassinolide on activity of enzymes of oxidative metabolism of cucumber in peronosporousepiphytotia conditions. In Plant Growth and Development Regulators, Moscow; Khripach, V.A., Zhabinskii, V.N., de Groot, A.E., Eds.; Academic Press: Moscow, USA, 1997.
- Korableva, N.P.; Platonova, T.A.; Dogonadze, M.Z.; Evsunina, A.S. Brassinolide effect on growth of apical meristems, ethylene production, and abscisic acid content in potato tubers. Biol. Plant 2002, 45, 39–43.
- Hoshi, T.; Yamada, K.; Fuji, S.; Furuya, H.; Yoshizawa, Y.; Oh, K. Antifungal activity of brassinosteroid biosynthesis inhibitors yucaizol derivatives against Magnaportheoryzae. Can. J. Pure Appl. Sci. 2015, 9, 3333–3338.
- Hoshi, T.; Yamada, K.; Yoshizawa, Y.; Oh, K. Structure-activity relationship study for fungicidal activity of 1-(4-phenoxymethyl-2-phenyl-(1,3)dioxolan-2-ylmethyl)-1H-1,2,4-triazole derivatives against rice blast. J. Plant Prot. Res. 2015, 55, 383–388.
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254.
- Gao, W.; Long, L.; Zhu, L.F.; Xu, L.; Gao, W.H.; Sun, L.Q.; Liu, L.L.; Zhang, X.L. Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703.
- Ali, S.S.; Gunupuru, L.R.; Kumar, G.S.; Khan, M.; Scofield, S.; Nicholson, P.; Doohan, F.M. Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol. 2014, 14, 227.
- Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.R.; Vera-Cruz, C.; Kikuchi, S.; Höfte, M. Brassinosteroids antagonize gibberellin-and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846.
- Skoczowski, A.; Janeczko, A.; Gullner, G.; Tóbias, I.; Kornas, A.; Barna, B. Response of brassinosteroid-treated oilseed rape cotyledons to infection with the wild type and HR-mutant of Pseudomonas syringae or with P. fluorescence. J. Therm. Anal. Calorim. 2010, 104, 131–139.
- Schumacher, K.; Chory, J. Brassinosteroid signal transduction: Still casting the actors. Curr. Opin. Plant Biol. 2000, 3, 79–84.
- Konstantopoulos, K. Editorial Hot Topic: Molecular biology-pathophysiology of inflammation and autoinflammation. Curr. Drug Target Inflammation Allergy 2005, 4, 1–39.
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential anti-inflammatory agents: Current status and future perspectives. J. Phytopharmacol. 2015, 4, 121–125.
- Alché, L.E.; Michelini, F.M. Antiherpetic and Anti-Inflammatory Activities of Novel Synthetic Brassinosteroids Analogs. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Pereira-Netto, A.B., Ed.; Bentham Science Publishers: Curitiba-PR, Brazil, 2012; pp. 72–83.
- Moreno-Anzurez, N.E.; Marquina, S.; Alvarez, L.; Zamilpa, A.; Castillo-España, P.; Perea-Arango, I.; Torres, P.N.; Herrera-Ruiz, M.; García, E.R.D.; García, J.T.; et al. A Cytotoxic and Anti-inflammatory Campesterol Derivative from Genetically Transformed Hairy Roots of Lopeziaracemosa Cav. (Onagraceae). Molecules 2017, 22, 118.
- Esposito, D.; Tuazon, M.; Henderson, G.C.; Komarnytsky, S.; Raskin, I. Brassinosteroid enhances C57BL/6J mice treadmill endurance. FASEB J. 2012, 26, 1121–1128.
- Raskin, I.; Esposito, D.; Komarnytsky, S.; Rathinasabapathy, T.; Rojo Castillo, L. Methods of Producing and using Brassinosteroids to Promote Growth, Repair, and Maintenance of Skeletal Muscle and Skin. U.S. Patent AU2011343970A1, 21 June 2012.
- Quiñones, J.P.; García, Y.C.; Curiel, H.; Covas, C.P. Microspheres of chitosan for controlled delivery of brassinosteroids with biological activity as chemicals. Carbohydr. Polym. 2010, 80, 915–921.
- Ferrari, I.; Michelini, F.; Berra, M.; Alche, L.; Aguinaga, H.; Berra, A. In vitro and in vivo Anti-Adenovirus and Anti-Inflammatory Activities of a New Synthetic Brassinosteroid Analogue. Inves. Ophthalmol. Vis. Sci. 2009, 50, 3098.
- Shen, X.; Hong, F.; Nguyen, V.A.; Gao, B. IL-10 attenuates IFN- K-activated STAT1 in the liver: Involvement of SOCS2 and SOCS3. FEBS J. 2000, 480, 132–136.
- He, C.; Liu, Z.; Huang, J.; Liu, T. Therapeutic effect of 28-homobrassinolide on leukotriene synthesis in leukemia cells. Cytotechnology 2017.
- Song, L.; Qu, D.; Zhang, Q.; Jiang, J.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol esters attenuate hepatic steatosis in rats with nonalcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017, 7, 41604.
- Athithan, V.; Ramesh, R.; Srikumar, K. 28-Homocastasterone: A Novel Dietary Phyto Keto Oxysterol Modulating Testicular Steroid Metabolism AndLxrMrna Expression in Diabetic Rat. Int. J. Pharm. Pharm. Sci. 2018, 10, 162–167.
- Pillay, S.; Byrne, H.M.; Maini, P.K. Modeling angiogenesis: A discrete to continuum description. Phys. Rev. E. 2017, 95, 012410.
- Panibrat, O.V.; Zhabinskii, V.N.; Khripach, V.A. Anticancer Potential of Brassinosteroids. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 389–406.
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072.
- Akhtar, J.; Tiwari, V.; Oh, M.J.; Kovacs, M.; Jani, A.; Kovacs, S.K.; Valyi-Nagy, T.; Shukla, D. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Investigative ophthalmology & visual science. Investig. Opthalmology Vis. Sci. 2008, 49, 4026–4035.
- Singh, R.P.; Agarwal, R. Tumor angiogenesis: A potential target in cancer control by phytochemicals. Curr. Cancer Drug Targets. 2003, 3, 205–217.
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis—A potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345.
- Pietras, R.J.; Weinberg, O.K. Antiangiogenic steroids in human cancer therapy. Adv. Access Pub. 2005, 2, 49–57.
- Ferrara, N.; Winer, J.; Burton, T.; Rowland, A.; Siegel, M.; Phillips, H.S.; Terrell, T.; A Keller, G.; Levinson, A.D. Expression of vascular endothelial growth factor does not promote transformation but confers a growth advantage in vivo to Chinese Hanster Ovary cells. J. Clin. Investig. 1993, 91, 160–170.
- D’Angelo, G.; Struman, I.; Martial, J.; Weiner, R. Activation of mitogen activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kd N-terminal fragment of prolactin. Proc. Natl. Acad. Sci. USA 1995, 92, 6374–6378.
- Eckhardt, S.G. Angiogenesis inhibitors as cancer therapy. Hosp. Pract. 1999, 34, 63–84.
- Akhter, S.; Nath, S.K.; Tse, C.M.; William, J.; Zasloff, M.; Donowitz, M. Squalamine, a novel cationic steroid, specifically inhibits the brushborder Na+/H+ exchanger isoform NHE3. Am. J. Physiol. Content 1999, 276, 136–144.
- Bhardwaj, R.; Sharma, I.; Kanwar, M.; Handa, N.; Kapoor, D. Current Scenario of Applications of Brassinosteroids in Human Welfare. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Pereira-Netto, A.B., Ed.; Bentham ebooks: Brazil, 2012; pp. 3–15. Available online: (accessed on 9 April 2020).
- Michelini, F.M.; Lombardi, M.G.; Bueno, C.A.; Berra, A.; Sales, M.E.; Alché, L.E. Synthetic stigmasterol derivatives inhibit capillary tube formation, herpetic corneal neovascularization and tumor induced angiogenesis: Antiangiogenic stigmasterol derivatives. Steroids 2016, 115, 160–168.
- Hoffmannová, L. A Study of Molecular and Cellular Activities of Brassinosteroids and their Derivatives. Available online: (accessed on 5 April 2020).
- Bajguz, A.; Bajguz, A.J.; Tryniszewska, E.A. Recent advances in medicinal applications of brassinosteroids, a group of plant hormones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 33–49.
- Onatskiy, N.M.; Marchenko, A.I.; Mikhina, L.V. Technical Report for Evaluation of Mutagenic Activity of Epibrassinolide (Active Ingredient of Epin) in Ames Test, Chromosome Aberrations and in Micronuclear Tests; Scientific Research Centre of Toxicologie and Hygienic Regulation of Biopreparations of Russia: Serpukhov, Russia, 1997.
- Howell, W.M.; Keller, G.E.; Kirkpatrick, J.D.; Jenkins, R.L.; Hunsinger, R.N.; McLaughlin, E.W. Effects of the plant steroidal hormone, 24-epibrassinolide, on the mitotic index and growth of onion (Allium cepa) root tips. Genet. Mol. Res. 2007, 6, 50–58.
- Sondhi, N.P.; Bhardwaj, R.; Kaur, S.; Kumar, N.; Singh, B. Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its antigenotoxicity employing Allium cepa chromosomal aberration assay. Plant Growth Regul. 2008, 54, 217–224.
- Sondhi, N.; Bhardwaj, R.; Kaur, S.; Chandel, M.; Kumar, N.; Singh, B. Inhibition of H2O2-induced DNA damage in single cell gel electrophoresis assay (comet assay) by castasterone isolated from leaves of Centellaasiatica. Health 2010, 2, 595.
- Moghadasian, M.H. Pharmacological properties of plant sterols in vivo andin vitro observations. Life Sci. 2000, 67, 605–615.
- Schroepfer, G.J. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554.
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757.
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175.
- Lukatkin, A.S.; Kashtanova, N.N.; Duchovskis, P. Changes in maize seedlings growth and membrane permeability under the effect of epibrassinolide and heavy metals. Russ. Agric. Sci. 2013, 39, 307–310.
- Khripach, V.; Altsivanovich, K.; Zhabinskii, V.; Samusevich, M. Method for Decreasing Cholesterol Level in Blood. U.S. Patent US6998397B2, 14 February.
- Kitron, A.; Pergamentz, R. Brassinosteroids for use in treating prostatic hyperplasia and androgenic alopecia. U.S. Patent WO2010064242A1, 10 June 2010.
- Statsenko, E.A.; Korolevich, M.P.; Seregkina, T.V.; Paramonova, N.A.; Ostapenko, V.A.; Ryibkina, I.L. Methods of correction of lipid metabolism in athletes. Voennaya Medicina (Mil. Med.) 2008, 9, 102–104.
- Statsenko, E.A. Prophylactic and Correction of Functional State Among Athletes of High Qualifying Categories under Training Process. Ph.D. Thesis, Federal Scientific Center of Physical Culture and Sport, Moscow, Russia, 2013.
- Mohan, R.; Heyman, R.A. Orphan nuclear receptor modulators. Curr. Top. Med. Chem. 2003, 3, 1637–1647.
- Lehmann, M.; Koolman, J. Ecdysteroid receptors of the blowfly Calliphoravicina: Partial purification and characterization of ecdysteroid binding. Mol. Cell. Endocrinol. 1988, 57, 239–249.
- Smagghe, G.; Decombel, L.; Carton, B.; Voigt, B.; Adam, G.; Tirry, L. Action of brassinosteroids in the cotton leaf worm Spodopteralittoralis. Insect Biochem. Mol. Biol. 2002, 32, 199–204.
- Richter, K.; Adam, G.; Vorbrodt, H.M. Inhibiting effect of 22S,23S-homobrassinolide on the moult of the cockroach Periplanetaamericana (L.) (Orthopt, Blattidae). J. Appl. Èntomol. 1987, 103, 532–534.
- Spindler, K.D.; Spindler-Barth, M.; Turberg, A. Action of brassinosteroids on the epithelial cell line from Chironomus tentans. Zeitschrift für Naturforschung C 1992, 47, 280–284.
- Lehmann, M.; Vorbrodt, H.M.; Adam, G.; Koolman, J. Antiecdysteroid activity of brassinosteroids. Experientia 1988, 44, 355–356.
- Sobek, L.; Bohm, G.A.; Penzlin, H. Ecdysteroid receptors in last instar larvae of the wax moth Galleria mellonella L. Insect Biochem. Mol. Biol. 1993, 23, 125–129.
- Davison, G.P.; Restrepo, R.; Martinez, G.; Coll, F.; Leon, O.S. Effects of a brassinosteroid analogue to mosquito larvae. Ecotoxicol. Environ. Saf. 2003, 56, 419–424.
- Dinan, L.; Bourne, P.C.; Meng, Y.; Sarker, S.D.; Toletino, R.; Whiting, P. Assessment of natural products in the Drosophila melanogaster BII cell bioassay for ecdysteroid agonist and antagonist activities. Cell. Mol. Life Sci. 2001, 58, 321–342.
- Decombel, L.; Tirry, L.; Smagghe, G. Action of 24-epibrassinolide on a cell line of the beet armyworm, Spodopteraexigua. Arch. Insect Biochem. Physiol. 2005, 58, 145–156.
- Oh, K.; Kamada, H.; Yamada, K.; Yoshizawa, Y. Mosquito Larvicidal Activity of Triazole Type Brassinosteroid Biosynthesis Inhibitors. Int. J. Biosci. Biochem. Bioinform. 2016, 6, 114–120.
- Slama, K.; Lafont, R. Insect hormones—Ecdysteroids: Their presence and actions in vertebrates. Eur. J. Entomol. 1995, 92, 355–377.
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 1–30.
- Jones, A.; Pruessner, J.C.; McMillan, M.R.; Jones, R.W.; Kowalik, G.T.; Steeden, J.A.; Williams, B.; Taylor, A.M.; Muthurangu, V. Physiological adaptations to chronic stress in healthy humans–why might the sexes have evolved different energy utilisation strategies? J. Physiol. 2016, 594, 4297–4307.
- Sheiko, I.P.; Budevich, A.I.; Khripach, V.A.; Smuneva, V.K.; Lebedev, S.G. Method of Increasing the Fertility of the Bull Sperm-Producer. Pat. 10768, 31 March 2008.
- Esposito, D.; Komarnytsky, S.; Shapses, S.; Raskin, I. Anabolic effect of plant brassinosteroid. FASEB J. 2011, 25, 3708–3719.
