Endometriosis is a disease with both physical and psychological consequences. It can affect fertility and chances of having a baby as well as getting an education or a steady job, it can also reduce the quality of a woman’s social life and her physical activity.
- endometriosis
- biomarker
- diagnostic markers
- CA-125
- urocortin
- activin A
- follistatin
- microRNA
- urinary biomarkers
1. Introduction
Endometriosis is a progressive disease with features of chronic inflammation. According to some scientific studies, there is a link between an inflammatory process and oxidative stress which may contribute to the development of endothelial dysfunction [4]. Other research points to immunological dysfunction as an initiator of the disease [5]. However, although inflammatory mediators are upregulated and inflammatory cells are activated, a pre-existing inflammation may not contribute to the development of endometriosis [6].
It is speculated that the polygenetic and polyepigenetic hypotheses, which have several clinical implications, are feasible enough to elucidate changes in the endometrium, immunology and placentation. A typical, deep and cystic ovarian endometriosis is often described as clonal in origin and manifested by clinical heterogeneity of the lesions, which may be suggestive of initial chromosomal modifications. Expression of the genetic changes transmitted at birth could increase predisposition towards endometriosis. Bleeding, oxidative stress, body radiation and dioxins are regarded as additional factors for activation of this process.
Endometriosis is characterized by the presence of endometrial-like tissue outside the uterus. The incidence of endometriosis in women of reproductive age is between 6% and 10%. It is also estimated that endometriosis occurs in 21–47% of infertile women as well as in 71–87% of women experiencing chronic pelvic pain. Endometriosis is a major cause of markedly reduced quality of life in women suffering from this disease [10].
The most common symptoms of endometriosis are painful sexual intercourse (deep dyspareunia), pain before and/or during menstruation Progression of the disease does not correlate with the aggravation of the symptoms and none of them are specific. As a result of the improper verification process, the patient may be unnecessarily treated for diseases that may mimic the symptoms associated with other chronic pain-related disorders, e.g., irritable bowel syndrome and pelvic inflammatory disease [13,14]. In addition, women with endometriosis experience a number of non-clinical symptoms that include depression, fatigue, and the feeling of isolation.
Laparoscopy, an invasive method of examination, preceded by a transvaginal ultrasound and pelvic magnetic resonance imaging (MRI), is still regarded as the gold standard in the diagnosis of endometriosis [14]. Therefore, it is hoped that development of non-invasive diagnostic tools, such as ‘biomarkers’, could significantly reduce the time taken to diagnose endometriosis and enable monitoring the progression of the disease and the effectiveness of its treatment [13]. Replacing the invasive diagnostic methods with biomarkers which comply with the predetermined criteria, i.e., 94% sensitivity and 79% specificity, could be of considerable clinical usefulness [16,17].
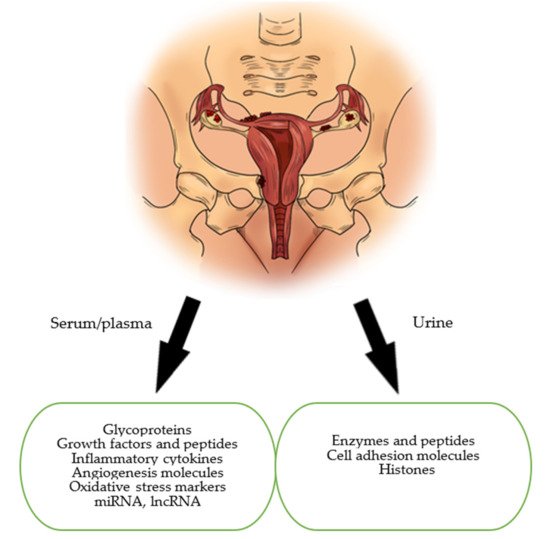
This paper’s aim is to present and discuss the current status of biomarkers of endometriosis in the serum and urine. In our review, we focused on the main groups of markers which are: glycoproteins, growth factors, peptides, immunological markers, markers of oxidative stress, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) (Figure 1).
We conducted a comprehensive literature review using electronic databases such as Pubmed, Science Direct and Google Scholar, and took into account articles published in English between 1988 and April 2021. Keywords such as: “biomarker”, “endometriosis”, “glycoproteins”, “urocortin”, “immunological markers”, “oxidative stress”, “microRNA”, “lncRNA”, “urinary biomarkers”, and various combinations of the above were used. Publications were selected if they related to the studies investigating potential biomarkers detected in women with endometriosis. In addition, we manually reviewed the references for each article to find potentially missed studies.
Inclusion criteria for the study selection: The samples of biomarkers could be collected from serum, plasma, whole blood, tissue, urine Randomized clinical trials, systemic reviews, meta-analyses Animal, human, in vitro studies.
Exclusion criteria: Case reports, conference summaries, comments Insufficient data Not accessible as a full-text article for review Language other than English Studies conducted on non-mammalian species.
2. Oxidative Stress Markers
The formation of reactive oxygen species (ROS) is a physiological process regulated by antioxidant defense mechanisms. Its significant role has been demonstrated in the inflammatory response of many diseases, including endometriosis [17]. It seems that as a result of retrograde menstruation, the endometrial cells and desquamated menstrual cells migrate into the peritoneal cavity, thereby inducing a chronic inflammatory response. This stimulates proinflammatory cytokines to activate immune cells such as granulocytes and macrophages, which are known to be capable of producing ROS [65].
These markers were distinguished in a meta-analysis from the Cochrane database, with carbonyls, showing a sensitivity of 94% and specificity of 51% in the detection of endometriosis (cut-off point < 14.9 mM), as well as in thiols, and they were regarded as substances particularly useful in the detection of pelvic endometriosis, showing a sensitivity of 73% and a specificity of 80% (cut-off < 396.44 mM). took into account 19 studies investigating oxidative stress markers in endometriosis. According to the analysis, the levels of glutathione peroxidase and superoxide dismutase significantly decreased in the severe stage of endometriosis, while the levels of lipid peroxide increased together with the severity of the disease [68]. However, other studies have shown that there is no correlation between endometriosis and the presence of oxidative stress markers [66].
Due to the multitude of factors regulating the level of oxidative stress, researchers emphasize the necessity for conducting further scientific studies to investigate if it is possible to use oxidative stress markers as diagnostic tests for endometriosis [17,69].
3. MiRNAs and lncRNAs
Its main function is the regulation of gene expression, it also affects processes of proliferation, differentiation, growth and apoptosis. MiRNAs are regulatory molecules that control expression of many genes and play key roles in many biological processes [70]. However, dysregulation of miRNA has been associated with many diseases, including endometriosis. MiRNA has brought a new perspective to the field of serum markers and become the subject of many research papers [71,72].
In 2020, Zhang et al., selected and tested specific types of miRNA: miR-134-5p, miR-197-5p, miR-22-3p, miR-320a, miR-494-3p, and miR-939-5p [72]. Two types of miRNA, i.e., miR-22-3p and miR-320a, were distinctly upregulated in the group of endometriosis patients in comparison to the control group. Moreover, a significant difference was noticed between the studied patients in stages Thus, it seems that these two types of miRNA could be potential biomarkers for endometriosis [72].
In 2016, Cosar et al., evaluated expression of different miRNAs and concluded that out of all the tested samples, the miR-125b-5b level was significantly upregulated in women with endometriosis [73]. Importantly, miR-125b has been observed to affect the levels of TNF-α, IL-6, and IL-1β, which belong to the group of inflammatory cytokines, and their concentration is elevated in women with endometriosis [74]. Another factor that affects inflammatory cytokine levels is miR-20a. In patients with endometriosis, miR-20a expression is downregulated, which leads to increased levels of aforementioned pro-inflammatory cytokines [40].
Moreover, Hudson et al., showed that miR-154-5p alone, or in combination with miR-196b, miR-378-3p, and miR-33a-5p, is linked to endometriosis [75].
Anastasiu et al. [40] as well as Bjorkman and Taylor [74] indicate the miR-200 family as a promising marker of endometriosis. The miR-200 family includes miR-200a, miR-200b and miR-141. Scientific studies have shown a reduced expression of miRNAs in a group of patients with endometriosis, and the studies cited by Anastasiu et al., are characterized by a sensitivity of 84.4% and s specificity of 66.7% [40,74].
In 2020, Moustafa et al., selected six miRNAs: miR-125b, miR-150, miR-342, 451a, miR-3613, Let-7b [75]. Regardless of the studied group’s diversity when it comes to the disease progression, menstrual cycle phase, different racial demographics and the presence of hormonal treatment, the obtained results showed a significant increase in the levels of miR-125b, miR-150, miR-342, 451a as well as a significant decrease in the levels of miR-3613 and Let-7b in patients with endometriosis. In addition, the authors observed differences in miRNAs for different stages of the disease; they also noticed that the phase of the menstrual cycle and hormone treatment had no effect on the measured values. One of the most important conclusions of this research is that endometriosis can be distinguished from other gynecological pathologies on the basis of miRNAs [75].
lncRNAs, a member of the RNA family, is considered as a marker of endometriosis. lncRNAs are molecules longer than 200 nucleotides which, even though they do not code for proteins, can regulate gene expression directly or indirectly by affecting the expression of miRNAs [76]. whose levels showed a significant dysregulation in the group of patients with endometriosis. Researchers also identified the lncRNA molecule TC0101441 whose increased level correlated with infertility, chronic pelvic pain, and recurrence of the disease [40].
MiRNAs as well as lncRNAs are very attractive diagnostic markers due to their lower complexity, tissue specificity, lack of known post-translational modifications and stability in blood, urine or tissues [77]. Researchers have summarized their studies on miRNA with a conclusion that it is an endometriosis marker with an average value of sensitivity of 86% and specificity of 88%, while in the case of lncRNA the sensitivity was 89.7% and specificity was 73.2% [78,79]. Although the results are promising, research into miRNA and lncRNA remains a new field of study and requires confirmation and further investigation [40,72,78].
4. Urinary Biomarkers in Endometriosis
According to the currently available scientific knowledge, biomarkers for endometriosis can also be detected in urine. Great advantages of using a urinary test in the diagnosis of endometriosis include its low cost, non-invasiveness and the fact that a urine sample can be collected by the patient herself. However, the reliability of laboratory techniques and changing levels of the urinary biomarker during the menstrual cycle are limitations of the test.
Enolase I (NNE) is an enzyme detected in urine and has been studied as a biomarker in patients with endometriosis. The urinary NNE expression corrected for the creatinine ratio (NNE-Cr) was significantly higher in patients with endometriosis. They suggest that it may have diagnostic value when combined with the serum CA-125. However, this area of interest requires further research investigating patients with a broader spectrum of endometriosis
The urinary VDBP levels corrected for creatinine (VDBP-Cr) expression were elevated in patients with endometriosis. VDBP-Cr was only different in the luteal phase of the cycle in women with endometriosis compared to the control group, which may be a limitation of the study. More in-depth evaluation of VDBP across the spectrum of endometriosis, especially in the luteal phase, is needed. An important aspect of further research is to determine the role, if any, that VDBP plays in endometriosis [81].
With the use of proteomic techniques such as MALDI-TOF MS (matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) and time-of-flight analyzer (TOF)), different peptide markers were described in the urine of women with endometriosis and compared with the controls. The preovulatory peptide mass of 1767.1 showed a sensitivity of 75% and a specificity of 85%. The luteal peptide mass of 1824.3 These results are promising but require further validation in large populations presenting all stages of endometriosis.
Another protein obtained from urine and considered as a biomarker for endometriosis is cytokeratin 19 (CK 19). [87] used proteomic techniques and thus demonstrated the presence of CK-19 in urine in women with endometriosis, comparing with a control group. In this survey, the study group is not big enough to conclusively specify the value of CK-19 as biomarker for endometriosis. It is necessary to perform more large-scale studies involving more patients.
Proestling et al. [88] investigated the usefulness of cell adhesion molecules such as sVCAM-1, sICAM-1, E-selectin and P-selectin as urinary biomarkers in the diagnosis of endometriosis. However, they found no significant differences in their urinary levels between women with and without endometriosis.
Chen and et al. [89] conducted a study to identify novel protein biomarkers that can be used to diagnose endometriosis. For the first time, histone 4 was identified as a potential biomarker for endometriosis with a sensitivity of 70% and specificity of 80%. This study may provide a new direction in the search for the most appropriate biomarker for endometriosis.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10132762
