Chemical heat storage is one of the most promising alternatives for thermal energy storage (TES) due to its high energy density, low energy loss, flexible temperature range, and excellent storage duration. Mg-based materials are pretty suitable for the heat storage application. In the hydrogen storage area, Mg-based materials are promising candidates due to the large abundant reserve in the crust, the light weight of Mg element, and high hydrogen storage capacity.
- thermal energy storage
- Mg-based materials
- hydrogen storage
- chemical heat storage
1. Introduction
|
Renewable Source |
Max. Power (TW) |
Percentage of Total Solar Energy |
|---|---|---|
|
Total surface solar [7] |
85,000 |
100% |
|
Desert solar [6] |
7,650 |
9% |
|
Ocean thermal [8] |
100 |
0.12% |
|
Wind [9] |
72 |
0.08% |
|
Geothermal [10] |
44 |
0.05% |
|
River hydroelectric [11] |
7 |
0.008% |
|
Biomass [6] |
7 |
0.008% |
|
Open ocean wave [12] |
7 |
0.008% |
|
Tidal wave [13] |
4 |
0.003% |
|
Coastal wave [14] |
3 |
0.003% |
2. Chemical Fundamentals

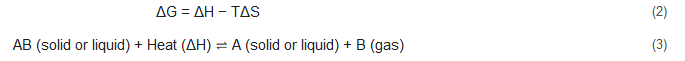
|
Heat Storage Method |
Materials |
Specific Heat (kJ/kg/K) |
Energy Density (GJ/m3) |
Working Temperature (°C) |
Reference |
|---|---|---|---|---|---|
|
Sensible heat |
Rock |
1.30 |
n.a. |
200−300 |
[23] |
|
Concrete |
0.85 |
n.a. |
200−400 |
[23] |
|
|
Mineral oil |
2.60 |
n.a. |
200−300 |
[1] |
|
|
Carbonate salts |
1.80 |
n.a |
450−850 |
[24] |
|
|
Latent heat |
KNO3/KCl |
1.21 |
n.a. |
320 |
[1] |
|
E117 (commercial) |
2.61 |
0.25 |
117 |
[1] |
|
|
A164 (commercial) |
n.a. |
0.46 |
164 |
[1] |
|
|
AlSi12 |
1.04 |
1.51 |
576 |
[1] |
|
|
Na2CO3 |
n.a. |
0.70 |
854 |
[23] |
|
|
Chemical heat |
Calcium carbonate |
n.a |
4.40 |
800−900 |
[25] |
|
Iron carbonate |
n.a. |
2.60 |
180 |
[26] |
|
|
Metal hydrides |
n.a. |
4.00 |
200−500 |
[27] |
|
|
Magnesium oxide |
n.a. |
3.30 |
250−400 |
[1] |
|
|
Hydroxides |
n.a. |
3.00 |
500 |
[27] |
n.a.: Not available.
3. Different Types of Mg-Based Materials for Thermal Energy Storage

|
Compound |
Reaction |
Material Energy Density |
Reaction Temperature (°C) |
|---|---|---|---|
|
Ammonia [30] |
NH3 + ΔH ↔ 1/2N2 + 3/2H2 |
67 kJ/mol |
400–500 |
|
Methane/water [27] |
CH4 + H2O ↔ CO + 3H2 |
n.a. |
500–1000 |
|
Hydroxides [27] |
Ca(OH)2 ↔ CaO + H2O |
3 GJ/m3 |
500 |
|
Calcium carbonate [25] |
CaCO3 ↔ CaO + CO2 |
4.4 GJ/m3 |
800–900 |
|
Iron carbonate [26] |
FeCO3 ↔ FeO + CO2 |
2.6 GJ/m3 |
180 |
|
Metal hydrides [27] |
Metal xH2 ↔ metal yH2 + (x − y) H2 |
4 GJ/m3 |
200–500 |
|
Metal oxides (Zn and Fe) [31] |
e.g., 2-step water splitting using Fe3O4/FeO redox system |
n.a. |
2000–2500 |
|
Aluminium ore alumina [1] |
n.a. |
n.a. |
2100–2300 |
|
Methanolation–demethanolation [32] |
CH3OH ↔ CO + 2H2 |
n.a. |
200–250 |
|
Magnesium oxide [1] |
MgO + H2O ↔ Mg (OH)2 |
3.3 GJ/m3 |
250–400 |
n.a.: Not available.
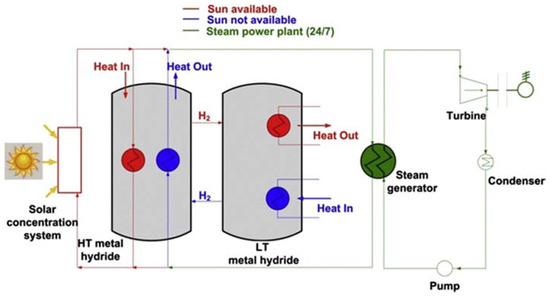
This entry is adapted from the peer-reviewed paper 10.3390/app8081375
References
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55.
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367.
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31.
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Peters, B.; Zidan, R. High performance metal hydride based thermal energy storage systems for concentrating solar power applications. J. Alloys Compd. 2015, 645, S374–S378.
- Qu, X.; Li, Y.; Li, P.; Wan, Q.; Zhai, F. The development of metal hydrides using as concentrating solar thermal storage materials. Front. Mater. Sci. 2015, 9, 317–331.
- Abbott, D. Keeping the energy debate clean: How do we supply the world’s energy needs? Proc. IEEE 2010, 98, 42–66.
- Mendoza, B. Total solar irradiance and climate. Adv. Space Res. 2005, 35, 882–890.
- Hermann, W.A. Quantifying global exergy resources. Energy 2006, 31, 1685–1702.
- Archer, C.L.; Jacobson, M.Z. Evaluation of global wind power. J. Geophys. Res. Atmos. 2005, 110.
- Pollack, H.N.; Hurter, S.J.; Johnson, J.R. Heat flow from the earth’s interior: Analysis of the global data set. Rev. Geophys. 1993, 31, 267–280.
- Milliman, J.; Farnsworth, K. Fluvial Discharge of Silicate to the Oceans: A Global Perspective; School of Marine Science, College of William and Mary: Gloucester Point, VA, USA, 1999.
- Panicker, N.N. Power resource estimate of ocean surface waves. Ocean Eng. 1975, 3, 429–439.
- Ray, R.; Eanes, R.; Chao, B. Detection of tidal dissipation in the solid earth by satellite tracking and altimetry. Nature 1996, 381, 595–597.
- Quayle, R.G.; Changery, M.J. Estimates of coastal deepwater wave energy potential for the world. In OCEANS 81; IEEE: Piscataway, NJ, USA, 1982; pp. 903–907.
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396.
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283.
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.W.; Jensen, T.R. Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 2017, 10, 1645.
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical heat storage for high temperature applications—A review. Green 2013, 3, 113–123.
- Felderhoff, M.; Bogdanovic, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344.
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345.
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553.
- Bogdanović, B.; Ritter, A.; Spliethoff, B. Active MgH2-Mg systems for reversible chemical energy storage. Angew. Chem. Int. Ed. 1990, 29, 223–234.
- Herrmann, U.; Kearney, D.W. Survey of thermal energy storage for parabolic trough power plants. J. Sol. Energy Eng. 2002, 124, 145–152.
- Reilly, H.; Kolb, G. Evaluation of Molten Salt Power Tower Technology Based on Experience at Solar Two; Report No. SAND2001-3674; Sandia National Laboratories: Albuquerque, New Mexico, 2001.
- Kubota, M.; Yokoyama, K.; Watanabe, F.; Hasatani, M. Heat releasing characteristics of CaO/CaCO3 reaction in a packed bed for high temperature heat storage and temperature up-grading. In Proceedings of the 8th International Conference on Thermal Energy Storage (Terrastock 2000), Stuttgart, Germany, 28 August–1 September 2000.
- Visscher, K. Solarcombi systems, heading for 100% solar fraction. Presented at the VSK Exhibition, Utrecht, The Netherlands, 2004.
- Hahne, E. Thermal energy storage: Some views on some problems. In Proceedings of the 8th International Heat Transfer Conference, San Francisco, CA, USA, 17–22 August 1986; pp. 279–292.
- Aochi, A.; Tadokoro, T.; Yoshida, K.; Kameyama, H.; Nobue, M.; Yamaguchi, T. Economical and technical evaluation of ut-3 thermochemical hydrogen production process for an industrial scale plant. Int. J. Hydrogen Energy 1989, 14, 421–429.
- Alefeld, G. Energiespeicherung durch heterogenverdampfung (I). Wärme 1975, 81, 89.
- Lovegrove, K.; Luzzi, A.; Kreetz, H. A solar-driven ammonia-based thermochemical energy storage system. Sol. Energy 1999, 67, 309–316.
- Steinfeld, A.; Sanders, S.; Palumbo, R. Design aspects of solar thermochemical engineering—A case study: Two-step water-splitting cycle using the Fe3O4/FeO redox system. Sol. Energy 1999, 65, 43–53.
- Shiizaki, S.; Nagashimga, I.; Iwata, K.; Hosoda, T.; Kameyama, H. Development of plate fin reactor for heat recovery system using methanol decomposition. In Proceedings of the 8th International Conference on Thermal Energy Storage (Terrastock 2000), Stuttgart, Germany, 28 August–1 September 2000.
- Rönnebro, E.; Whyatt, G.; Powell, M.; Simmons, K.; Fang, Z.; White, R. Reversible metal hydride thermal energy storage for high temperature power generation systems. In Proceedings of the Concentrating Solar Power Program Review, Phoenix, AZ, USA, 23–25 April 2013; pp. 29–30.
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601.
- Shao, H.; He, L.; Lin, H.; Li, H.-W. Progress and trends in magnesium-based materials for energy-storage research: A review. Energy Technol. 2018, 6, 445–458.
- Li, J.; Xu, J.; Li, B.; He, L.; Lin, H.; Li, H.W.; Shao, H. Advanced sem and tem techniques applied in Mg-based hydrogen storage research. Scanning 2018, 2018.
- Shao, H. Heat modeling and material development of Mg-based nanomaterials combined with solid oxide fuel cell for stationary energy storage. Energies 2017, 10, 1767.
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and downsizing in Mg-based hydrogen storage materials. Catalysts 2018, 8, 89.
- Bogdanović, B.; Ritter, A.; Spliethoff, B.; Straβburger, K. A process steam generator based on the high temperature magnesium hydride/magnesium heat storage system. Int. J. Hydrogen Energy 1995, 20, 811–822.
- Bogdanović, B.; Spliethoff, B.; Ritter, A. The magnesium hydride system for heat storage and cooling. Z. Phys. Chem. 1989, 164, 1497–1508.
- Reiser, A.; Bogdanović, B.; Schlichte, K. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrogen Energy 2000, 25, 425–430.
- Friedlmeier, G.; Groll, M. Experimental analysis and modelling of the hydriding kinetics of Ni-doped and pure mg. J. Alloys Compd. 1997, 253, 550–555.
- Steiner, D.; Groll, M.; Wierse, M. Development and Investigation of Thermal Energy Storage Systems for the Medium Temperature Range; American Society of Mechanical Engineers: New York, NY, USA, 1995.
- Buchner, H.; Povel, R. The daimler-benz hydride vehicle project. Int. J. Hydrogen Energy 1982, 7, 259–266.
- Marty, P.; Fourmigue, J.-F.; De Rango, P.; Fruchart, D.; Charbonnier, J. Numerical simulation of heat and mass transfer during the absorption of hydrogen in a magnesium hydride. Energy Convers. Manag. 2006, 47, 3632–3643.
- Askri, F.; Jemni, A.; Nasrallah, S.B. Study of two-dimensional and dynamic heat and mass transfer in a metal–hydrogen reactor. Int. J. Hydrogen Energy 2003, 28, 537–557.
