Resveratrol (Res) is a well-known natural product that can exhibit important pharmacological activities such as antioxidant, anti-diabetes, anti-tumor, and anti-inflammatory. An evaluation of its therapeutic effects demonstrates that this naturally occurring bioactive compound can target different molecular pathways to exert its pharmacological actions. Transforming growth factor-beta (TGF-β) is an important molecular pathway that is capable of regulating different cellular mechanisms such as proliferation, migration, and angiogenesis. TGF-β has been reported to be involved in the development of disorders such as diabetes, cancer, inflammatory disorders, fibrosis, cardiovascular disorders, etc.
1. Resveratrol
From immemorial times, plant-derived natural compounds have been under attention in the treatment of different disorders such as inflammatory diseases, cancers, pulmonary diseases, metabolic disorders, neurological disorders (NDs) including Alzheimer’s disease (AD) and Parkinson’s disease (PD), infertility, and so on [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. Phytochemicals can exhibit beneficial actions against diseases due to their excellent pharmacological activities [
11,
12,
13,
14]. These benefits have resulted in extensive research into finding new natural compounds and revealing their potential mechanisms of actions [
15,
16,
17]. Resveratrol (Res) is a dietary phytochemical that has been reported to be efficacious treatment for various ailments by targeting diverse molecular pathways [
18,
19,
20,
21]. The role of Res in the treatment of chronic diseases was established in early 1990s when it was found that this phytochemical possesses significant cardioprotective benefits [
22]. This ascending trend toward Res research led to the revelation of its significant biological and therapeutic activities. The first report about anti-tumor activity of Res dates back to 1997, when Jang and his colleagues reported its inhibitory effect on leukemia [
23].
Currently, Res can be derived from various plants including
Arachis hypogea,
Cassia sp.,
Eucalyptus sp.,
Morus rubra, and so on using a number of different isolation techniques [
24]. High-performance liquid chromatography is the best strategy [
25,
26,
27,
28]. Over the past decades, Res has been applied in the treatment of various diseases such as osteoarthritis [
29,
30,
31], NDs [
32], cancer [
33,
34,
35], diabetes [
36], cardiovascular diseases [
37], liver disorders [
38], and so on. An increasing amount of evidence is in agreement with the fact that Res affects different molecular pathways to exhibit its protective effects [
39,
40,
41]. Hence, the identification of these targets can promote further studies for investigating molecular pathways and the mechanisms of its therapeutic actions in depth. For instance, anti-inflammation is one of the most important biological effects of Res treatment. To function as an anti-inflammatory molecule, Res can effectively inhibit the activation of pro-inflammatory transcription factors such as nuclear factor-kappaB (NF-ĸB). It seems that the anti-inflammatory actions of Res are not only mediated via inhibitory actions on the NF-ĸB signaling pathway, but they also rely on its action as a PARP-γ agonist [
42]. The anti-inflammatory activities of Res are also characterized by decreased levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α), etc. [
43]. The production of pro-inflammatory lipid mediators from arachidonic acid can be mediated by the cyclooxygenase (COX) pathway. A number of anti-inflammatory drugs have been developed based on their inhibitory effect on COX-1 and COX-2 [
44,
45]. Res is capable of binding to the active site of COX-1 and thus causing anti-inflammatory effects. In addition to targeting inflammation, Res attaches to the active site of COX-2 to suppress cancer proliferation [
46,
47,
48,
49]. It is noteworthy that the inhibitory effect of Res on COX has been noted to follow a dose-dependent kinetics [
50].
Obesity is one of the challenges faced in today’s world. Res has demonstrated great potential in reducing weight and exerting anti-obesity activity. Res changes white adipose tissue (WAT) into brown adipose tissue (BAT), which in turn decreases weight and improves insulin resistance [
51]. The inhibitory action of Res on lipid accumulation leads to its effect on cardiovascular disorders. Res stimulates PARP-α/γ to activate ATP binding cassette (ABC) transporter A1/G1-mediated cholesterol efflux, resulting in a decrease in lipid accumulation and cholesterol levels. These effects can lead to a significant amelioration of atherosclerosis [
52]. Based on the effect of Res on amyloid-beta (Aβ), this plant-derived natural compound is of importance in treating NDs. For instance, Res is able to inhibit inflammation and the microglial activation caused by Aβ. This results in the alleviation of inflammation (down-regulation of TNF-α and IL-6) and a diminution in apoptosis (caspase-1 down-regulation) [
53]. The antioxidant activity of Res provides its protective effect during kidney injury. In rats exposed to nicotine, an increase occurs in oxidative stress markers via the down-regulation of glutathione. The administration of Res has been also correlated with improving the antioxidant defense system that protects renal cells against oxidative injury [
54]. A newly published study also demonstrates the effect of Res on stem cells. Res can stimulate stem cell function to ameliorate pancreatic injury such as fibrosis and apoptosis [
55]. Overall, these reports exhibit that Res has diverse therapeutic effects that have resulted in its extensive application in the treatment of various disorders [
56,
57,
58]. In the current review, we specifically focus on the therapeutic effects of Res mediated by its regulatory action on the transforming growth factor-β (TGF-β) signaling pathway.
2. Resveratrol and TGF-β Signaling Pathway
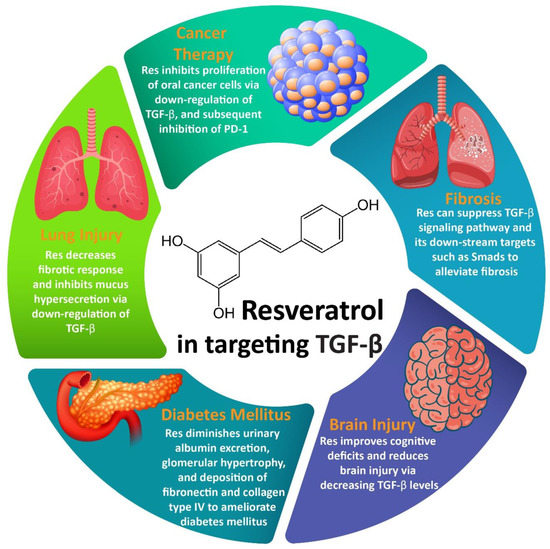
2.1. Resveratrol and Fibrosis
Pulmonary fibrosis (PF) is a common disorder of the lung that is characterized with hypoxemia, restrictive functional ventilatory disturbance, and chronic fibrosis. Clinical manifestations of PF include wheezing, difficulties in breathing, and dry coughs [
150]. The pathogenesis of PF is still not completely understood, but it appears that the TGF-β signaling pathway plays a significant role in PF development [
151]. Thus, the administration of Res may be an ideal strategy in the amelioration of PF, and different molecular pathways may be involved. Normally, microRNA (miR)-21 can induce PF via the activation of TGF-β signaling and providing Smad7 nuclear translocation. TGF-β provides a positive feedback loop, so TGF-β enhances the expression of miR-21 and AP-1. The administration of Res down-regulates the expression of miR-21 via inhibition of the MAPK/AP-1 axis. This leads to a diminution in TGF-β expression and inhibition of Smad7, resulting in the alleviation of PF [
152]. Accumulating data demonstrate that during the inhibition of fibrosis, Res affects the TGF-β signaling pathway via the modulation of miRs. Myocardial fibrosis (MF) is caused by the accumulation of collagen fibers, enhanced collagen content, and alteration in collagen composition. Systolic and diastolic functions of the heart can be negatively affected by MF [
153]. TGF-β is one of the key players regulating MF [
154]. The TGF-β/Smad7 axis can also contribute to the development of MF. The administration of Res can up-regulate the expression of miR-17, which in turn remarkably reduces levels of Smad7, leading to an improvement in MF [
155].
In addition to PF and MF, renal fibrosis (RF) can arise as a result of the activation of the TGF-β signaling pathway. It has been reported that the inhibition of the TGF-β signaling pathway by natural products such as bardoxolone and nimbolide is of importance in RF therapy [
156,
157]. It is worth mentioning that Res can target the TGF-β signaling pathway, thereby causing an amelioration of RF. In RF treatment, fibroblast–myofibroblast differentiation (FMD), EMT, and the proliferation of tubular epithelial cells (TECs) should be targeted. The administration of Res can disrupt Smad2/3 activation by TGF-β and consequently suppress the proliferation of TECs, FMD, and EMT [
158]. Increasing evidence demonstrates that EMT may be involved in renal fibrogenesis, and its activation can facilitate the development of RF [
159,
160,
161,
162]. Res is capable of suppressing EMT-mediated RF. It seems that TGF-β1 functions as an upstream mediator of EMT, and Res suppresses EMT and RF through inhibiting TGF-β1 [
163]. In fact, in the stimulation of anti-fibrotic activity, Res affects the proliferation and survival of fibroblasts. It has been shown that Res can stimulate apoptosis in fibroblasts and suppress their growth as well. An investigation of the molecular pathways demonstrates that in targeting fibroblasts, Res can suppress TGF-β and the Smad2/3/4 complex, and it can also upregulate Smad7 [
164].
It is worth mentioning that the anti-fibrotic activity of Res is dose-dependent, and using low doses is preferred as compared to higher doses. An experiment has evaluated the role of dose in the anti-fibrotic activity of Res. TGF-β induces fibrosis via formation of the Smad3/4 complex and subsequent stimulation of EMT. The administration of Res has been correlated with the deacetylation of Smad3 and Smad4 via sirtuin 1 (SIRT1). According to in vitro results, low doses of Res (5–20 mM) effectively exerted anti-fibrotic activity, while high doses (more than 40 mM) did not demonstrate any substantial anti-fibrotic activity. The in vivo findings are in line with in vitro results, so that low doses of Res (less than 25 mg/kg) improve fibrosis, while high doses of Res (more than 50 mg/kg) deteriorated the condition [
165]. This study confirms the dose-related toxicity of Res. Overall, these studies demonstrate that TGF-β can function as a key player in the development of fibrosis and Res can suppress the TGF-β signaling pathway and its downstream targets such as Smads to alleviate fibrosis [
166,
167].
The TGF-β signaling pathway contributes to the development of fibrosis in different vital organs of body such as the lung and heart. The interesting point to highlight is the possible epigenetic regulation of TGF-β by miRs in the development of fibrosis. Res is capable of suppressing miR and TGF-β interaction in fibrosis therapy. MiR-17 and miR-21 are two important miRs that contribute to the emergence of myocardial and pulmonary fibrosis via TGF-β induction. The regulation of TGF-β by miRs is suppressed upon Res administration. RF also occurs by the function of TGF-β and subsequent induction of EMT. The TGF-β/EMT axis is inhibited by Res to alleviate RF. It is noteworthy that in the amelioration of fibrosis, components of TGF-β signaling such as Smad7 and Smad4 can also be down-regulated. Therefore, TGF-β is a versatile agent in the amelioration of fibrosis.
2.2. Resveratrol and Cancer Therapy
Accumulating data exhibit that the TGF-β signaling pathway can regulate both the proliferation and metastasis of cancer cells, and its inhibition is a promising strategy in cancer therapy [
168,
169,
170,
171,
172,
173]. Metastasis is an increasing challenge in the effective treatment of cancer. Cancer cells are able to migrate into neighboring and distant tissues, demanding novel strategies in the inhibition of their metastasis. EMT is one of the mechanisms that can promote invasion via the transformation of static epithelial cells into migratory mesenchymal ones [
174]. A number of different molecular pathways have been recognized as regulators of EMT [
175,
176], and it has been found that TGF-β is capable of elevating migration via EMT induction. In breast cancer, TGF-β can stimulate EMT via Smad2 and Smad3 activation, leading to an increase in N-cadherin and vimentin levels, and a decrease in E-cadherin levels. The administration of Res suppresses the metastasis of breast cancer (under both in vitro and in vivo conditions) via the inhibition of TGF-β1 and down-regulation of Smad2 and Smad3 [
177]. TGF-β also contributes to the migration and malignant behavior of lung cancer. In addition to breast cancer, Res targets TGF-β to inhibit EMT in lung cancer. By suppressing levels of TGF-β, Res down-regulates the levels of vimentin and fibronectin, while it enhances E-cadherin levels, leading to an inhibition of EMT and metastasis of lung cancer cells [
178]. It is noteworthy that EMT induction enhances viability via the stimulation of cancer stem cell markers such as Bmi1 and Sox2. By inhibition of the TGF-β/Smad axis, Res not only inhibits EMT and migration, but also interferes with the proliferation and survival of cancer cells [
179]. So, Res can function as a potential modulator of EMT in cancer cells to negatively affect their proliferation and metastasis.
Accumulating data also show that Res is able to diminish levels of TGF-β that in turn, suppresses the development of renal carcinoma [
180]. These studies are in agreement with the fact that the inhibition of TGF-β by Res is of interest in suppressing tumor growth and metastasis [
181]. Moreover, a dual relationship has been found between TGF-β and programmed cell death-1 (PD-1). For instance, PD-1 overexpression is associated with the induction of TGF-β, and TGF-β can regulate PD-1 expression [
182,
183]. This dual relationship is of importance in cancer therapy. Res can suppress the proliferation of oral cancer cells via the down-regulation of TGF-β and subsequent inhibition of PD-1. L-thyroxine as a thyroid hormone can also modulate the anti-tumor activity of Res via regulating the TGF-β/PD-1 axis [
179].
Overall, the regulation of TGF-β by Res in cancer is of importance in terms of suppressing both migration and proliferation. The most well-known mechanism targeted by TGF-β is EMT, which can promote cancer metastasis. In addition, TGF-β can activate the signaling pathways such as PD-1 and Sox2 to ensure the growth and survival of cancer cells. Upon Res administration, TGF-β and its downstream targets are inhibited to pave the road for effective cancer therapy.
2.3. Resveratrol and Lung Injury
Injuries to vascular endothelium and alveolar epithelium by inflammatory factors can lead to the emergence of acute lung injury (ALI) [
184]. Infections are able to generate ALI and among them,
Pseudomonas aerogenosa,
Candidate albicans, and
staphylococcal enterotoxin B (SEB) are of importance [
185,
186,
187]. In the amelioration of SEB-mediated lung injury, Res can target the TGF-β signaling pathway. Res can down-regulate the expression of miR-193a to inhibit TGF-β2 and TGFβR3, thus resulting in a decrease in levels of inflammatory cytokines and T cell infiltration [
188]. The enhanced level of TGF-β has been associated with the development of asthma and lung injury [
189]. In fact, the administration of Res may alleviate lung injury and asthma via decreasing levels of TGF-β [
190]. Chronic obstructive pulmonary disease (COPD) is one of the most common disorders of lung tissue. Cigarette smoking is the most well-known reason for COPD [
191]. Pulmonary inflammation, airflow obstruction, and remodeling are features of COPD [
192]. Chronic inflammation can result in the development of COPD, and TGF-β has been found to play an important role in the pathogenesis of this disease [
193,
194]. Therefore, based on the modulatory impact of Res on TGF-β, the administration of this naturally occurring compound can be advantageous in the amelioration of COPD. It was also found that Res can decrease fibrotic response and inhibit mucus hypersecretion via the down-regulation of TGF-β [
195].
It seems that via the regulation of TGF-β, Res is capable of reducing inflammation in lung and preventing the development of pathological events such as ALI, COPD, and asthma. Interestingly, Res inhibits inflammation via reducing the infiltration of cytokines and T cells. COPD is also emerged via pulmonary inflammation and fibrosis. Based on the effect of Res on TGF-β and subsequent decrease in fibrotic response and mucus hypersecretion, it can be beneficial in the treatment of COPD.
2.4. Resveratrol and Brain Injury
Cerebral hemorrhage is a leading cause of brain injury and vasospasm [
196]. This malignancy results in ischemic/reperfusion and the induction of apoptosis in cancer cells [
197,
198]. The TGF-β signaling pathway has been correlated with brain injury [
199]. Interestingly, the administration of Res was found to improve the blood–brain barrier (BBB) and inhibit apoptosis in neuronal cells. These protective effects of Res were found to be mediated via the inhibition of TGF-β-mediated ERK [
200]. Moreover, it was found that exposing rats to alcohol is associated with an increase in levels of cytokines such as TGF-β. An administration of Res (10 and 20 mg/kg) can significantly improve cognitive deficits and reduces brain injury via decreasing TGF-β levels [
201]. So, the alleviation of cognitive deficits and maintaining the integrity of BBB are functions of Res that can be mediated by TGF-β modulation.
2.5. Resveratrol and DM
During DM, microvascular complications can lead to hyperglycemia that accounts for the emergence of diabetic nephropathy (DN). Interestingly, an enhanced level of oxidative stress, renal polyol formation, protein kinase C induction, and activation of AMPK as well as the accumulation of advanced glycation end-products (AGEs) are responsible for DN [
202,
203]. TGF-β1 is considered as one of the potential pathways involved in the emergence of DN [
204]. A combination of Res and rosuvastatin (RSU) was found to be beneficial in the alleviation of DN via the down-regulation of TGF-β1 [
205]. The in vivo studies have also indicated that the administration of Res is a promising strategy in alleviating DN. It was observed that Res could diminish urinary albumin excretion, glomerular hypertrophy, and the deposition of fibronectin and collagen type IV to ameliorate DN. Moreover, an investigation of molecular pathways demonstrated that Res can alleviate TGF-β expression as well as the phosphorylation of Smad2 and Smad3 for DN alleviation (Figure 2) [
206]. The most important effect of Res during DN is reducing fibrosis, which can be mediated via TGF-β inhibition.
Figure 2. Regulation of TGF-β signaling by Res and its association with therapeutic effects.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines8080261