Bluetongue virus (BTV) is an arthropod-borne virus vectored by biting midges from the Culicoides genus. BTV infects both domestic and wild ruminants, and the infection induces different variations of pathogenicity, from asymptomatic infection to severe symptoms such as ecchymosis, cardiac lesions, and hemorrhages, especially in sheep. Since the 1950s, BTV has spread globally from Africa to Europe, Asia, North and South America. Further, periodic outbreaks of new serotypes cause high morbidity in livestock, often with significant mortality and consequently associate with substantial economic losses in the agricultural industry.
BTV belongs to the
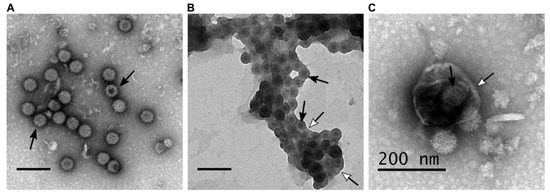
Reoviridae family, which are characterised by a non-enveloped icosahedral capsid. The ten segments of the BTV double-stranded RNA genome encode seven structural proteins (VP1, VP2, VP3, VP4, VP5, VP6 and VP7) and six non-structural proteins (NS1, NS2, NS3, NS3A, NS4 and NS5) [
1,
2]. During infection, VP2 and VP5, which form the outer capsid, are responsible for BTV attachment and entry into cells via the endocytic pathway. Within early and late endosome, pH-induced conformational changes of VP2 and VP5 facilitate membrane penetration of the core [
3], which is then released into the cytoplasm. The core is composed of an inner capsid (comprised of VP3 and VP7), containing the transcription complexes that are constituted by VP1 (RNA-dependent RNA polymerase), VP4 (capping enzyme), VP6 (helicase/RNA packaging) and the dsRNA segments [
4,
5]. The non-structural proteins fulfil essential functions within the virus life cycle, but do not form part of the virion. NS1 is necessary for BTV replication and selectively enhances viral protein synthesis [
6]. It also undergoes polymerisation producing large tubules in infected cells, whose functional consequence is unknown [
7]. NS2 is responsible, and sufficient, for the generation of viral inclusion bodies (VIBS), large globular structures in cytoplasm of infected cells. NS2 acts as a scaffold in the cytoplasm, recruiting positive sense ssRNA transcripts, the transcription complex components, and inner capsid proteins into VIBs. VIBs are, therefore, the sites of virus replication and are believed to be the site of viral core assembly, as inner capsid protein VP3 and VP7 have been found inside VIBs [
8]. The outer capsid protein VP5 is also observed in the VIBs, suggesting that the first layer of the outer capsid could be assembled in the VIBs [
9,
10]. In contrast, the assembly of the VP2 protein does not happen in the VIBs [
11], and the localisation of core maturation and VP2 assembly remains to be determined. However, it is likely that BTV uses the cell cytoskeleton for virus particles maturation, as it was previously demonstrated that VP2 is able to interact with the vimentin intermediate filaments [
12]. The NS3 protein is the only glycoprotein containing membrane domains synthesised by BTV. NS3 is believed to be synthesised at the endoplasmic reticulum (ER), and transits through the Golgi apparatus, where it becomes glycosylated, before reaching the plasma membrane [
13,
14]. NS3 is the major regulatory protein of virus maturation, trafficking and egress [
15,
16,
17,
18]. Here, we review the recent advances made on the routes of BTV egress and discuss the different mechanisms, and the role played by NS3.