The cactus family is a diverse plant group, native to the Americas. Most of the species included have very variable stem shapes, with singular geometric forms, one of the characteristics why they are considered interesting or even bizarre plants. Cacti vegetative organs have adaptations to arid ecosystems, while possessing showy and beautiful flowers. This set of characteristics has made them very popular among specialists and amateurs. In this entry we address some of the generalities of cacti, focusing on the particular modifications present in their flowers. These peculiarities make them an interesting model to study the development and evolution of these structures.
- flower development
- floral shoot
- flower evolution
- cacti evolution
- evo-devo
- flower organ identity
1. Introduction
Cactaceae is a eudicot family within the order Caryophyllales and have been organized into five subfamilies: Leuenbergerioideae, Pereskioideae, Maihuenioideae, Opuntioideae and Cactoideae [1][2][3]; see Figure 1 for a simplified phylogeny. The family diverged quite recently, at approximately 35 Mya, placing Cactaceae as a late arrival in angiosperm history. It is thought that the majority of species diversification occurred during the late Miocene, ≈10–5 Mya [4]. Cactaceae comprises between 1438 to 1870 species belonging to about 120 to 130 genera [5][6][7]. Its greatest species richness is concentrated primarily in Mexico, with secondary centers in the southwestern Andean region and in eastern Brazil [8]. Although cacti occur naturally only in the New World, the epiphytic cactus Rhipsalis baccifera, also occurs in tropical Africa, Madagascar and on islands of the Indian Ocean [7][9].
This succulent plants are distributed across a wide variety of ecosystems, from deserts to rainforests, and have a highly specialized vegetative axis where most species seem to lack leaves as these are highly reduced (microscopical) and modified (nonfunctional) leaves (reduced to spines). CAM photosynthesis (Crassulacean Acid Metabolism) is undertaken in succulent stems that, in turn, exhibit a wide variety of shapes (cylinder, barrel shape, flattened) and sizes [4][5]. All species of the family bear characteristic spine clusters (i.e., areoles), representing short shoots with leaves transformed into spines already at the stage of primordia. This characteristic is a true synapomorphy of the entire family [7]. Stem structure and metabolic modifications are considered adaptations to hydric stress and aridity [4][10].
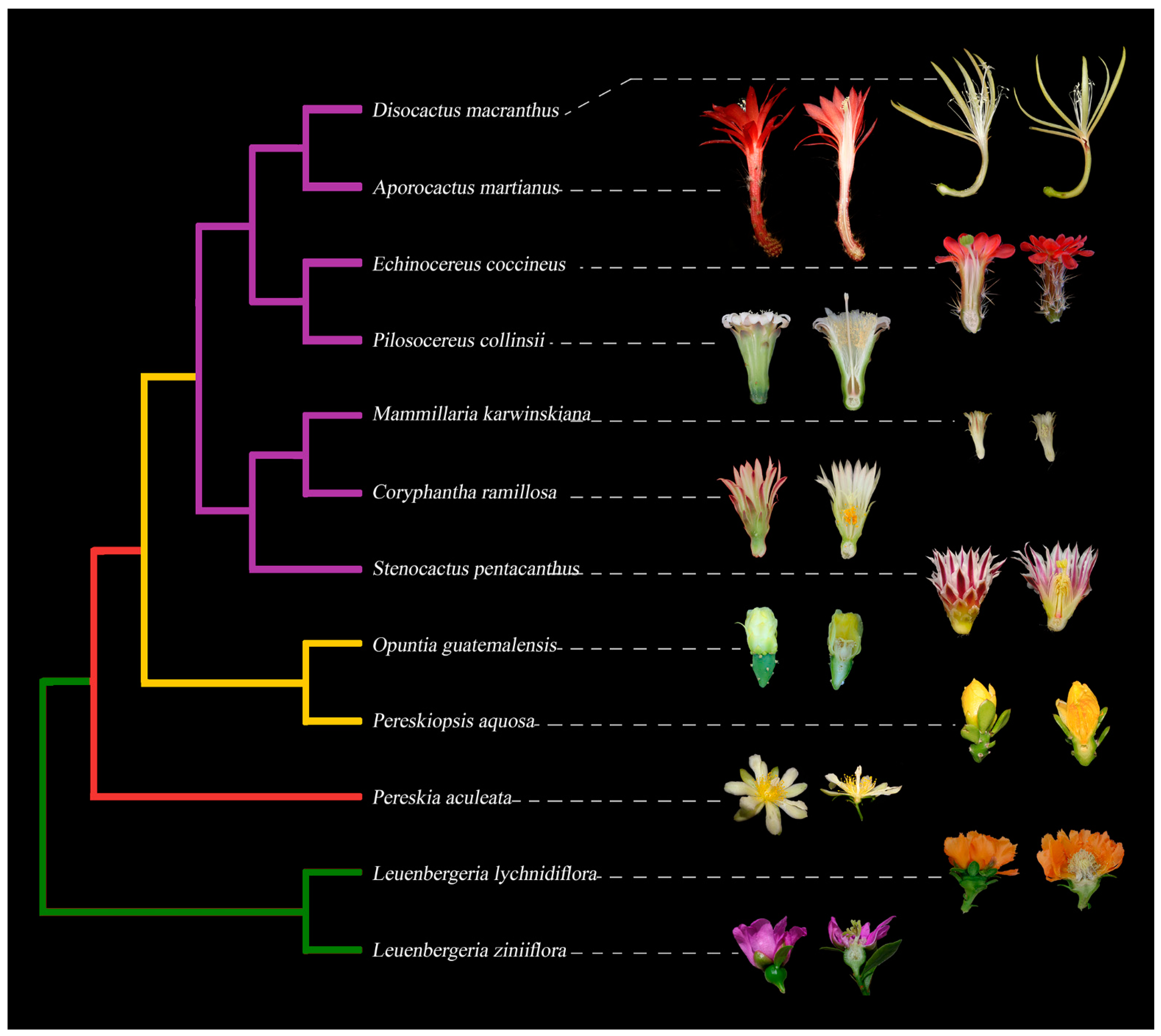
Figure 1. Simplified phylogeny of Cactaceae with representative flowers in longitudinal sections, exemplifying the diversity in floral morphology across the family. Leuenbergerioideae (green clade): Leuenbergeria zinniiflora, flowers with a hypanthium-like structure covering the ovary, with green sepal-like whorls and purple petal-like whorls; L. lychnydiflora, with green sepal-like whorls and orange petal-like whorls. Pereskioideae (red clade): P. aculeata, flowers with receptacle with areoles covering the superior ovary. Opuntioideae (yellow clade): Pereskiopsis aquosa, flowers with areoles and laminar leaves over the pericarpel; Opuntia guatemalensis, flowers with a succulent and thick pericarpel, foliar organs becoming tepaloid towards the apex. Cactoideae (purple clade): S. pentacanthus, C. ramillosa and M. karwinskiana showing a very reduced campanulate receptacle and a reduced pericarpel without areoles, spines and bracts; P. collinsii, showing a campanulate receptacle, without spines in the pericarpel and decurrent podaries; E. coccineus, showing a campanulate receptacle with a green spiny pericarpel and a red perianth; A. martianus showing a long tubular receptacle with bracts, spines and a red pericarpel, as well as a red perianth; D. macranthus, with a campanulate receptacle and a long tube, with a very reduced pericarpel and areoles, with a yellow perianth.
The Cactaceae are also quite diverse regarding plant habit, as its members include trees, like the North American genus Cephalocereus, dwarfs like Blossfeldia liliputana, giants like Carnegiea gigantea, epiphytes like the Selenicereus species in North America or Schlumbergera in South America, as well as geophytes like the Mexican genus Ariocarpus [11]. Their growth form has been classified in: globose, cylindric, columnar and depressed globose [12].
The diversity of forms observed in cacti stems could be surpassed by floral diversity (Figure 2). Flowers with different colors, sizes, shapes, scents, number of elements of perigon, time of anthesis, etc., can be observed across the family. This floral diversity has been associated with different pollination syndromes, as some cacti are mostly pollinated by bees (i.e, Pereskia and Opuntia), others by bats (i.e, Pachycereus), birds (i.e, Disocactus, Cleistocactus) and sphingid moths (i.e, Epiphyllum) [13]. A mixed pollination syndrome -where more than one pollinator type can be involved- has also been observed in particular species within the family [13][14][15].
From a morphological perspective, flowers are defined as short shoots that carry reproductive organs. This term acquires particular meaning in Cactaceae, since the flower is interpreted as a branch with a perianth at the tip, with all reproductive organs embedded within the branch, thus giving way to a structure that has been called a “flower shoot” [11][16] because the ovary and the floral tube are covered by an axial tissue called the pericarpel [17].

Figure 2. Examples of the diversity of flower shoots and stems in Cactaceae. (a) Magenta flower of Disocactus eichlamii. (b) Cephalocereus senilis, columnar cacti covered by hair-like structures. (c) Flower of Myrtillocactus geometrizans pollinated by a bee. (d) Orange flower of Opuntia tomentosa. Note the flat stem. (e) Flower and fruit of Lophocereus marginatus. (f) Flower of columnar cacti Pilosocereus aff. leucocephalus. (g) Opuntia cochenillifera, with stigma and androecium exerted. (h) burgundy floral bud with green pericarpel of Cephalocereus polylophus (i) stems of Aporocactus flagelliformis growing over a tree in the cloud forest. (j) Red flower of a globose Echinocereus coccineus. (k) Stenocactus pentacanthus; variegated flower and unique stem (l) globose depressed stems of Lophophora williamsii (m) Escobaria dasyacantha with yellowish flowers (n) Flower and fruit of Lophocereus marginatus (o) White flowers with green stigma of Cochemiea estebanensis (p) globose stem of Echinocactus platyacanthus (q) Disocactus macranthus (r) Epiphytic Disocactus x kimnachii with large red flowers (s) White flower of Stenocereus pruinosus (t) Epiphytic Selenicereus glaber (u) Purple flower of Echinocereus rayonesensis (v) Orange flower in Echinocereus scheeri whit green pericarpel (w) A group of globose Mammillaria geminispina, with distinctive white spines.
Given the diversity in form and rapid speciation process, the taxonomic classification in Cactaceae is quite complex, since many different names have been proposed for particular species and genera. To resolve these conflicts molecular phylogenetic analyses have been incorporated in taxonomic studies. Chloroplast DNA markers and some nuclear DNA markers have helped resolve taxonomic conflicts at the species, genus, tribe, subfamily and family levels [18][19][8][20][21][22]. These phylogenetic studies support the evolutionary relationships within the family and with its outgroups [10]. Members of the cactus family have shown to be excellent model systems to understand evolutionary processes such as stress hydric adaptations [10]. However, cacti are also great model species to study the evolution of vegetative and reproductive unit development as different clades possess unique modifications in stem, leaves, roots and flower shoots.
2. Cactus Flower Development
According to Boke [23], the development of the so-called flower shoot in Cactaceae is similar to that in many other plants, and the most significant differences are observed in pistil and, in particular, carpel development. In Cactaceae, the androecium has numerous stamens (multistaminated), which initiate in a uniform and prominent ring primordium, on which the stamens arise in a centrifugal succession [17][24][25][26]. In the Caryophyllales, the multistaminate androecium presents morphological similarities to the Paeoniaceae subfamily and Dilleniidae subclass, and it is thus considered to be an ancestral feature in the Caryophyllales [27][26].
Generally, cacti ovaries develop multiple ovules [28], but Pereskia aculeata develops fewer than five ovules [27][29]. Ovule development in cacti exhibits similar characteristics to other members of the Caryophyllales, such as the hook-like shape of the carpels, which is also reported in Phytolacca, as well as the ovules’ primordium at the base of the ovary, such as that seen in Phytolacca and Tetragonia [17]. Another feature is the secondary augmentation of the ovules along the cross-zone, as in Trianthema and Mesembryanthemum [17]. The structure that has no homologs in other members of Caryophyllales is the pericarpel, a tissue that covers the ovary and seemingly is ontogenetically related to the stem. The ontogeny of this structure, together with the perianth, deserves further attention due to its likely complex evolution.
3. The Pericarpel
The term pericarpel was designated by Buxbaum [17] referring to the tissue which surrounds the ovary (carpels) in cacti. This tissue can be green and photosynthetic. In many cacti species like S. undatus (dragon fruit) the pericarpel is covered by bracts or scales, which can be considered as true leaves due to the presence of axillary buds [16]. The pericarpel is characterized by the presence of areoles (modified axillary buds) and spines (modified leaves), which are also present in cacti stems [11][23]. In Opuntia, the pericarpel bears stomata and areoles on its surface [30]. In addition, the presence of mucilaginous cells, druses and parenchyma cells resembles those present in the stem [60,61]. Based on these features, it has been suggested that the pericarpel has an axial origin; in other words, the pericarpel is a vegetative tissue originating from a vegetative meristem within the areole. Due to the axial–appendicular nature of the tissue, Mauseth [11][16] and most contemporary cacti specialists refer to this unique and remarkable structure as a “flower shoot”.
This concept of the flower shoot or the assembly between vegetative and floral tissues has been widely accepted in cacti [11][16][31]. Although the anatomical evidence supports the idea of an axial origin in the pericarpel, the homology of the pericarpel with the stem needs the conduction of formal studies, to determine the developmental and evolutionary origin of this structure. Therefore, we propose some hypotheses of the ontogenetic origin of the pericarpel and possible genes involved in the determination of this complex structure. The ontogeny of this structure, together with the perianth, deserves further attention due to its likely complex evolution.
4. The Perianth
Cactaceae represent a highly derived clade with an increased number of petaloid sepals [32], which are indeterminate and polymerous [28], developing in a centripetal order [17] with a spiral phyllotaxis [33]. Ronse De Craene [34] considered that the perianth in Cactaceae is formed by sepals and not by petals. Due to this, he denominated this structure as “petaloid sepals” and considered them nonhomologous to the petals present in species such as A. thaliana or A. majus. As we mentioned above, the perianth in the cactus family has been considered to have a gradation from bracts to sepals to petals [9][17] or bracts to tepals [17][35].This phenomenon has never been addressed in detail from a developmental or an ontogenetic perspective, but studying it would be instrumental to determine whether the perianth of a seemingly sepaloid origin present in this family is a product of a reduction in the petal whorl or if there is, in fact, a morphological gradation related to the differential expression of transcription factors directing perianth development that favors the interpretation of a transition from bracts to sepals and petals within a cactus flower (Figure 4).
5. Perspectives
The conspicuous characteristics observed in flower shoots of cacti, such as the presence of multiple perianth series with a possible sepaloid/bracteoid origin and the existence of a unique structure termed the pericarpel, make cacti flower shoots an exciting model to further our insights into the different developmental venues that underlie flower diversification in angiosperms.
The ABC model of flower development, proposed a generic mechanism for flower determination that has been a useful conceptual framework to test for the genetic basis of homologous organs in many angiosperms. Recent studies in a diverse set of flowering plants have suggested that several variations of the Arabidopsis/Antirrhinum model exist, particularly with respect to the perianth whorls [36][37][38][39]. In Cactaceae, the apparent lack of homology of perianth structures with respect to Arabidopsis, as well as the unique pericarpel structure, suggests that cacti could represent an additional variation on a theme involving synorganization between reproductive and vegetative tissues; this must be further investigated through comparative gene expression and developmental genetic studies that could help ascertain the conserved functions and unique variations present in a structure whose morphological and histological identities need further study.
This entry is adapted from the peer-reviewed paper 10.3390/plants10061134
References
- Hunt, D.R.; Taylor, N.P.; Charles, G.. The new cactus lexicon; DH Books: Port, UK, 2006; pp. 900.
- Mayta, L.; Molinari-Novoa, E.A.; L’intégration Du Genre Leuenbergeria Lodé Dans Sa Propre Sous-Famille, Leuenbergerioideae Mayta & Mol. Nov., subfam. nov. Succulentopi 2015, 12, . Succulentopi 2015, 12, 6–7, .
- Reto, N.; Urs Eggli, U.; Disintegrating Portulacaceae: A new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. TAXON 2010, 59, 227-240, 10.1002/tax.591021.
- Arakaki, M.; Christin, P.-A.; Nyffeler, R.; Lendel, A.; Eggli, U.; Ogburn, R.M.; Spriggs, E.; Moore, M.; Edwards, E.J.; Contemporaneous and recent radiations of the world's major succulent plant lineages. Proceedings of the National Academy of Sciences 2011, 108, 8379-8384, 10.1073/pnas.1100628108.
- Hunt, D.R.; Taylor, N.P.; Charles, G. The New Cactus Lexicon; DH Books: Port, UK, 2006; ISBN 9780953813445.
- Nyffeler, R.; Eggli, U. An up-to-Date Familial and Suprafamilial Classification of Succulent Plants. Bradleya 2010, 2010, 125–144.
- Caryophyllales . Caryophyllales. Retrieved 2021-6-19
- Hernández-Hernández, T.; Hernández, M.H.; De-Nova, J.A.; Puente, R.; Eguiarte, L.E.; Magallón, S.; Phylogenetic relationships and evolution of growth form in Cactaceae (Caryophyllales, Eudicotyledoneae). American Journal of Botany 2010, 98, 44-61, 10.3732/ajb.1000129.
- Anderson, E. F.. The cactus family; Timber Press: Portland, OR, USA, 2001; pp. 776.
- Guerrero, P.C.; Majure, L.C.; Cornejo-Romero, A.; Hernández-Hernández, T.; Phylogenetic Relationships and Evolutionary Trends in the Cactus Family. Journal of Urban Health 2018, 110, 4-21, 10.1093/jhered/esy064.
- Mauseth, J.D.; Structure-Function Relationships in Highly Modified Shoots of Cactaceae. Annals of Botany 2006, 98, 901-926, 10.1093/aob/mcl133.
- Vázquez-Sánchez, M.; Terrazas, T.; Arias, S.; El hábito y la forma de crecimiento en la tribu Cacteae (Cactaceae, Cactoideae). Botanical Sciences 2012, 90, 97, 10.17129/botsci.477.
- Schlumpberger, B.O.; A survey on pollination modes in cacti and a potential key innovation. Evolution of Plant-Pollinator Relationships 2012, , , 10.1017/cbo9781139014113.011.
- Boris O. Schlumpberger; Ernesto I. Badano; DIVERSITY OF FLORAL VISITORS TO ECHINOPSIS ATACAMENSIS SUBSP. PASACANA (CACTACEAE). Haseltonia 2005, 11, 18-26, 10.2985/1070-0048(2005)11[18:dofvte]2.0.co;2.
- Gorostiague, P.; Ortega-Baes, P.; How specialised is bird pollination in the Cactaceae?. Plant Biology 2015, 18, 63-72, 10.1111/plb.12297.
- Mauseth, J.D.; Many Cacti have Leaves on Their “Flowers”. Cactus and Succulent Journal 2016, 88, 60-65, 10.2985/015.088.0202.
- Buxbaum, F. The Flower. In Morphology of Cacti; Kurtz, E.B., Jr., Ed.; Abbey Garden Press: Pasadena, CA, USA, 1953; pp. 94–170.
- Nyffeler, R.; Phylogenetic relationships in the cactus family (Cactaceae) based on evidence from trnK/ matK and trnL-trnF sequences. American Journal of Botany 2002, 89, 312-326, 10.3732/ajb.89.2.312.
- Bárcenas, R.T.; Yesson, C.; Hawkins, J.A.; Molecular systematics of the Cactaceae. Cladistics 2011, 27, 470-489, 10.1111/j.1096-0031.2011.00350.x.
- Korotkova, N.; Borsch, T.; Arias, S.; A phylogenetic framework for the Hylocereeae (Cactaceae) and implications for the circumscription of the genera. Phytotaxa 2017, 327, 1-46, 10.11646/phytotaxa.327.1.1.
- Cruz, M.A.; Arias, A.; Terrazas, T.; Molecular phylogeny and taxonomy of the genus Disocactus ( Cactaceae ), based on the DNA sequences of six chloroplast markers. Willdenowia 2016, 46, 145-164, 10.3372/wi.46.46112.
- Tapia, H.J.; Bárcenas-Argüello, M.L.; Terrazas, T.; Arias, S.; Phylogeny and Circumscription of Cephalocereus (Cactaceae) Based on Molecular and Morphological Evidence. Systematic Botany 2017, 42, 709-723, 10.1600/036364417x696546.
- Boke, N.H.; Developmental Morphology and Anatomy in Cactaceae. BioScience 1980, 30, 605-610, 10.2307/1308111.
- Payer, J.-B.. Traité D’Organogénie Comparée de La Fleur; Masson: Amsterdam, The Netherlands, 1857; pp. -.
- H. Boke, N.H.; Anatomy and Development of the Flower and Fruit of Pereskia pititache. American Journal of Botany 1963, 50, 843-858, 10.1002/j.1537-2197.1963.tb10655.x.
- Leins, P.; Erbar, C.. Putative Origin and Relationships of the Order from the Viewpoint of Developmental Flower Morphology. In Caryophyllales: Evolution and Systematics; Behnke, H.-D., Mabry, T.J., Eds.; Springer: Berlin Heidelberg: Berlin/Heidelberg, Germany, 1994; pp. 303–316.
- Norman H. Boke; Ontogeny and Structure of the Flower and Fruit of Pereskia aculeata. American Journal of Botany 1966, 53, 534-542, 10.1002/j.1537-2197.1966.tb07368.x.
- Hofmann, U.; Flower Morphology and Ontogeny. Caryophyllales 1993, , , 10.1007/978-3-642-78220-6_7.
- Leuenberger, B.E.. Pereskia (Cactaceae); In Memoires of New York Botanical Garden, Eds.; NYBG Press: New York, NY, USA, 1986; pp. -.
- Fuentes-Pérez, M.; Terrazas, T.; Arias, S; Anatomía Floral de Cinco Especies de Opuntia (Opuntioideae, Cactaceae) De México. Polibotánica 2009, 27, 89-102, .
- Mauseth, J.D.; Rebmann, J.P.; Rodrigues-Machado, S.; Extrafloral Nectaries in Cacti. Cactus and Succulent Journal 2016, 88, 156-171, 10.2985/015.088.0401.
- Ronse De Craene, L.P.; Reevaluation of the perianth and androecium in Caryophyllales: implications for flower evolution. Plant Systematics and Evolution 2013, 299, 1599-1636, 10.1007/s00606-013-0910-y.
- Brockington, S.; Alexandre, R.; Ramdial, J.; Moore M.; Crawley, S.; Dhingra, A.; Hilu, K.; Soltis, D.E.; Soltis, P.S.; Phylogeny of the Caryophyllales Sensu Lato: Revisiting Hypotheses on Pollination Biology and Perianth Differentiation in the Core Caryophyllales. International Journal of Plant Sciences 2009, 170, 627-643, 10.1086/597785.
- Ronse De Craene, L.P.; Are Petals Sterile Stamens or Bracts? The Origin and Evolution of Petals in the Core Eudicots. Annals of Botany 2007, 100, 621-630, 10.1093/aob/mcm076.
- Bravo-Hollis, H.. Las Cactáceas de Mexico; Universidad Nacional de México: Mexico City, Mexico, 1978; pp. -.
- Nakamura, T.; Fukuda, T.; Nakano, M.; Hasebe, M.; Kameya, T.; Kanno, A.; The modified ABC model explains the development of the petaloid perianth of Agapanthus praecox ssp. orientalis (Agapanthaceae) flowers. Plant Molecular Biology 2005, 58, 435-445, 10.1007/s11103-005-5218-z.
- Kanno, A.; Nakada, M.; Akita, Y.; Hirai, M.; Class B Gene Expression and the Modified ABC Model in Nongrass Monocots. The Scientific World Journal 2006, 7, 268-279, 10.1100/tsw.2007.86.
- Soltis, D.E.; Chanderbali, A.S.; Kim, S; Buzgo, M; Soltis, P.S.; The ABC Model and its Applicability to Basal Angiosperms. Annals of Botany 2007, 100, 155-163, 10.1093/aob/mcm117.
- Irish, V.; The ABC model of floral development. Current Biology 2017, 27, R887-R890, 10.1016/j.cub.2017.03.045.
