The experimental production of complex structures resembling mammalian embryos (e.g., blastoids, gastruloids) from pluripotent stem cells in vitro has become a booming research field. Since some of these embryoid models appear to reach a degree of complexity that may come close to viability, a broad discussion has set in with the aim to arrive at a consensus on the ethical implications with regard to acceptability of the use of this technology with human cells. The present text focuses on developmental autonomy of embryoids which is an aspect of great ethical relevance and must receive increased attention in new legal regulations, but which has not been icluded in the recently issued ISSCR Guidelines.
- stem cells
- embryoids
- blastoids
- gastruloids
- blastocyst
- expanded potential stem cells
- development
- morphogenesis
- self-organization
- ethics
https://encyclopedia.pub/user/revision/main/draft/7d48c7eb6375e15fc9f0a925f93af1c3
Autonomy in Stem Cell-Derived EmbryoidsSubjects: Biology
Definition
The experimental production of complex structures resembling mammalian embryos (e.g., blastoids, gastruloids) from pluripotent stem cells in vitro has become a booming research field. Since some of these embryoid models appear to reach a degree of complexity that may come close to viability, a broad discussion has set in with the aim to arrive at a consensus on the ethical implications with regard to acceptability of the use of this technology with human cells. The present text focuses on developmental autonomy of embryoids which is an aspect of great ethical relevance and must receive increased attention during the preparation of new legal regulations, but which has not been included yet in the recently issued ISSCR Guidelines.
1. Introduction
The experimental production of complex structures resembling mammalian embryos (or parts of them) from stem cells in vitro has recently become a rapidly growing research field (for reviews, see [1,2,3,4,5,6,7,8,9]). Depending on the degree of structural complexity, various types of such embryoids can be distinguished. For many years it has been known that structures which only vaguely resemble real mouse embryos in an early germ layer stage can develop from pluripotent stem cells (PPSC) in ascites or in suspension culture, i.e., the so-called embryoid bodies (for illustrations, see Figures 1 and 13 in [10]). During the last few years, however, numerous studies have been published on stem cell-derived constructs which resemble mouse and human embryos or embryo parts much more closely. Remarkably, this is emerging as a novel, booming research field, and various new terms have now been introduced to address these various entities (summarized and illustrated in [5]): blastoids (resembling blastocysts), polarized embryo-like structures, gastrulating embryo-like structures, gastruloids, post-implantation amniotic sac embryoids, and asymmetric human epiblast. A general term proposed for such human embryoids is SHEEFs (Synthetic Human Entities with Embryo-like Features) [11].
As a result of the wealth of recent observations and publications, it is now widely realized that the morphogenetic potential of PPSCs should be of ethical concern when dealing with human cells, and it has become a topic to ask whether and where a line may be drawn between embryoids and real embryos, if any. The need for discussions on the ethical aspects of this peculiar stem cell potential for embryoid formation was indeed already seen many years ago, at the time when the first publications on human PPSCs appeared [12,13,14]; however, this aspect started to receive broader attention only much later [11,15]. In recent years, studies with improved methodologies for stem cell culturing have shown that embryoids can now be formed more regularly, and that some of these constructs can develop into complex structures which may come close to resembling, e.g., primitive streak or even more advanced stages (gastruloids), in the mouse model as well as in the human [18,19,20,21,22,23,24,25,26,27,28,29,30]. It was then asked whether these technical advances would be reason enough to question the validity of the limit so far set in many countries, for research with human embryos (or stem cell-derived embryo-like entities?) at the primitive streak/14-day stage [31,32]. Specifically with regard to stem cell-derived embryoids, a broad discussion has been started by various institutions, with the aim to arrive at a consensus on whether new legislation should be initiated, and whether such new laws could be more liberal as to permit producing even more complete and advanced stage human embryoids for research [6,33,34,35,36], and which of the various embryoid models, and cultivation up to what stages, might be considered ethically permissible.
Any call for such changes in legislation should of course not be based only on the prospect of the new research possibilities but must consider all ethically relevant aspects connected with this new technology. In such considerations, an aspect often addressed is whether the degree of artificiality of embryoid constructs should be decisive; not the functionality (principal viability) of the “end product”, but rather how it came into existence, i.e., in a “natural” or an “artificial” way (various intricate manipulations, „embryo engineering“; for the type of manipulations used and for references to the voluminous literature, see the full article 10.3390/cells10061461). Concerning ethical implications, such embryo-engineering approaches naturally focus our attention on the act of purposefully constructing these entities. Would this very act of “engineering” have any bearings on the dignity we have to ascribe to the emerging entities? Although this view is defended by some authors, it is refuted by others who argue that the functionality (possible viability) of the construct should be seen as the major point of concern [54,55].
2. Autonomy in the Morphogenesis of Stem Cell-Derived Embryoids
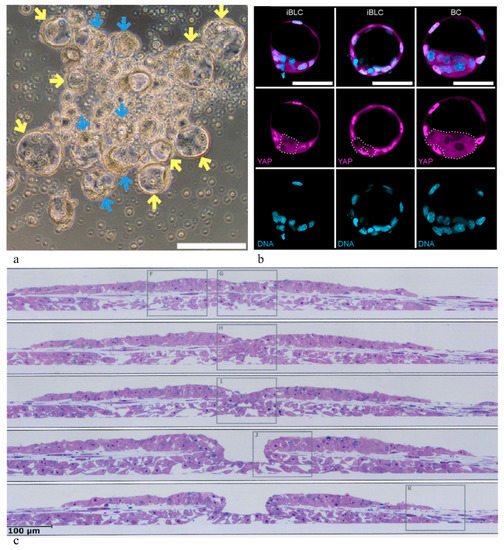
As experimental embryology has taught us, the development of structural order, specifically of a basic body plan, involves patterning processes that start with the formation of asymmetry centers, the elaboration of positional information and the initiation of spatially ordered gene activation cascades. The origin of the initial asymmetry signals in regular development is still a matter of discussion and is being investigated (for literature, see chapter 2 in the full article 10.3390/cells10061461). Recent research gives reason to reconsider theories on a significant role of segregation of cytoplasmic factors asymmetrically localized in the oocyte cytoplasm [67]. In stem cell cultures, however, such specific structural information must be missing: In contrast to early blastomeres, stem cells, even ES cells derived from early embryos, must be expected to have lost any oocyte-derived cytoplasmic asymmetry during passaging. In addition, it appears improbable that developmentally significant asymmetries of this type can be regained through reprogramming of fibroblasts, or at primed-to-naïve (and back) conversion of stem cells in culture as performed in some experimental approaches [71]. Nevertheless, remarkable self-organization processes have been and are being reported to occur in PPSC cultures, under certain conditions and even spontaneously, starting processes of this type independent of any specific embryo engineering maneuvers. Some examples are illustrated in Figure 1, e.g., blastocyst-like cysts recently described to sprout spontaneously in the mouse [71] (to be discussed more in detail further below). A particularly striking example are embryonic disc-like structures that have been observed already in 1996 to develop in dense cultures of marmoset monkey PPSCs (Figure 5 in [72]). Those authors described a primitive streak-like ingression center as well as amnion-like and yolk sac-like structures. Gastrulation-like ingression centers were also described to develop in rhesus monkey PPSC colonies (Figure 1c [73]). Anterior–posterior axis initiation has also been found to occur spontaneously in suspension cultures of mouse stem cell aggregates, a seminal observation that was received by many as a surprise [19] (gastruloid formation [20]). As has been discussed earlier [14], it seems to be significant for what is going on in cultures of stem cells that the asymmetry signals which probably govern morphogenetic patterning (the development of embryonic axes) in vivo can obviously be replaced by surrogate asymmetries in stem cell cultures, e.g., asymmetries in cell–cell and matrix densities that are always present in cultures and which arise stochastically [14].
Figure 1. Embryoid structures autonomously developing in stem cell cultures. (a,b) Blastocyst-like cysts sprouting from a colony of mouse PPSCs: (a) morphology; (b) composing cell types of induced blastocyst-like cysts (iBLCs) in comparison with a blastocyst (BC). Embryoblast cells: magenta (YAP); DNA: light blue (from Kime et al. [71], with permission). (c) Embryonic disc-like colony formed spontaneously in rhesus monkey PPSC culture, showing a crater mimicking gastrulation-like cell ingression (serial sections) (from [73], with permission).
Nevertheless, in many of the recent embryoid formation experiments using stem cells, it was found to be helpful to use a degree of “engineering” by providing some type of spatial information, e.g., to combine the various types of cells in an appropriate polar arrangement [23], or to apply physical constraints or specially engineered matrices [37,49,74]. They do not seem to be indispensable, however, as demonstrated by the observations on spontaneous/autonomous initiation of stem cell-derived embryoid morphogenesis just mentioned.
3. Spontaneous Budding of Blastocyst-Like Cysts in Stem Cell Cultures
In contrast to embryo-engineering experiments, a striking phenomenon of spontaneous morphogenesis has been described in recent studies by Kime et al. [71,75], i.e., the formation of blastoids, so-called “induced blastocyst-like cysts (iBLCs)”. This development, remarkably, did not require specific physical manipulations but occurred spontaneously by a budding-like process, under their conditions (Figure 1a,b). Of note, considerable numbers of such iBLCs were produced in each culture vessel. In their detailed analysis of the composing cell types, the authors emphasized the presence of three types of cells, autonomously taking typical positions in the iBLCs, i.e., trophoblast-like, primitive endoderm-like, and embryoblast-like cells, the latter showing properties of “expanded/extended potential stem cells (EPSCs)” or “2C-like cells” (discussed further below). In these experiments, Kime et al. [71,75] employed a methodology which they had previously developed and which allows conversion of primed state mouse PPSCs into the naïve state and v.v. with appropriate media supplements [76].
3.1. Implantation Potential of Blastocyst-Like Cysts, and Endometrial Response
A remarkable observation in the work by Kime et al. [71] was that such iBLCs initiated early parts of an implantation cascade after transfer to a uterus in this mouse model, i.e., they elicited an implantation-like response (decidualization) in the endometrium and formed extraembryonic tissues. This was comparable to the initial phase of implantation events as seen with normal blastocysts transferred as a control. However, the extraembryonic membranes formed by iBLCs were grossly underdeveloped and disorganized, and the anlage of the embryo proper did not proceed on to differentiate a basic body plan. Instead, the whole artificial “conceptuses” became resorbed later on. In addition to blastocysts, other controls were similarly transferred to a uterus: mouse epiblast stem cell clusters, and embryoid bodies. These latter two controls did not implant nor elicit a decidual reaction, thus providing evidence that the iBLCs had shown a remarkable blastocyst-like behavior, i.e., a degree of specificity in their interaction with the receptive endometrium. This was obviously due to the fact that the iBLCs possessed trophoblast, in contrast to epiblast stem cell clusters and embryoid bodies. Overall, the observations by Kime et al. on implantation potential were quite comparable to results obtained with other stem cell-derived blastocyst-like constructs, as presented by Li et al. [39].
3.2. Extended/Expanded Potential Stem Cells, 2C-Like Cells
With regard to the developmental potentiality of the reported iBLCs, an important point appears to be that they possessed, in addition to trophoblast-like and primitive endoderm-like cells, a population of cells exhibiting molecular properties of so-called “extended/expanded potential stem cells” (EPSCs) or “2C-like cells”, interesting sub-types of PPSCs that just recently started to receive attention in a number of labs [9,51,52,78,79,80,81,82,83,84,85,86,87,88].
Investigations on EPSCs gained momentum around 2015, with one of the first publications from the Torres-Padilla group on this peculiar subspecies of PPSCs [78]. These authors described a method of how “2C-like cells” (which had previously been found to occur spontaneously in stem cell cultures in small numbers [89]) can be induced to arise more frequently in vitro through down-regulation of the chromatin assembly activity of CAF-1. Ishiuchi et al. [78] reported that the EPSCs (“2C-like cells”) resembled blastomeres isolated from two-cell stage embryos, not only with regard to gene expression patterns but also to the capacity to reactivate transcription of endogenous retroviruses, as well as to the embryo-forming capacity gained during reprogramming by nuclear transfer to oocyte cytoplasm.
EPSCs are much smaller than two-cell blastomeres, and like other PPSCs they cannot be expected to possess any axis pre-information provided via asymmetrical segregation of cytoplasmic determinants derived from an oocyte/zygote. As discussed before [14], self-organized early embryonic pattern formation can apparently be initiated in colonies of stem cells by surrogate signals, e.g., asymmetries in cell densities or physical constraints, as well as by the structure of the extracellular matrix. This is in agreement with computer modelings of pattern formation processes in development (the Turing/Meinhardt model) [14]. Such surrogate signals can be decisive for pattern formation in stem cell colonies in vitro (e.g., in the system used by Warmflash et al. [74] as discussed earlier [91]). For example, the type of extracellular matrix or of feeder layers was previously found to be critical for the formation of gastrulation-like craters (as shown in Figure 1c) in rhesus monkey PPSC colonies [92]. If the potential of EPSCs would be tested by, e.g., transferring the cells into an empty zona pellucida (providing a neutral environment excluding locally acting external asymmetry signals), it could be seen whether autonomous morphogenesis would be possible or impossible in this case [55]. Effects of the addition of an artificial local source of morphogen could be investigated. Such experiments could shed light on the question of what exactly the differences might be between a (totipotent) morula and a cluster of PPSCs/EPSCs formed under certain conditions. In order to determine how close the biological properties of EPSCs may come to those of blastomeres, it could appear interesting to study in various types of embryoids whether or not a regular primitive streak (and, thus, an incipient basic body plan) can be formed autonomously in vitro. It should be remembered that autonomous morphogenesis in marmoset monkey stem cell cultures was already reported, many years ago, to sometimes reach advanced stages, including primitive streak formation [72]. It would be of much interest to investigate the role of EPSCs in this type of morphogenesis in the marmoset monkey model [93]. Indeed, such experiments should not be performed in the human but with non-human primate PPSCs, in order to avoid any formation of a human basic body plan anlage in vitro [11,15,91].
4. Conclusions and Ethical Implications
Undifferentiated PPSCs are not zygotes or embryos. However, as discussed, groups (colonies) of PPSCs can gain developmental autonomy in the process that is now usually addressed as self-organization, either on an organ level (organoids) or an early embryo level (blastoids, various higher organization levels of embryoids), depending on the epigenetic state of the initiating stem cells and on the local conditions provided in culture. For the start of these pattern formation processes and the subsequent morphogenesis, very simple asymmetries are obviously sufficient, as can occur in cultures stochastically [14]. The recent progress with formation of various types of embryoids from stem cells shows that these constructs are now reaching impressive complexity, with mouse as well as with human cells; that relatively well-structured embryonic as well as extraembryonic tissue (trophoblastic shell, amnion, yolk sac) anlagen are formed; and that a primitive streak stage can be reached in vitro. The anterior part of the primitive streak is known to act as an organizer, instrumental in the formation of a basic body plan (discussed in [14]). Organizer action marks individuation; this is the last stage of development at which the formation of identical twins is possible. It was for this reason that the primitive streak stage was set as a limit even in relatively liberal legal rulings for the use of human embryos for research, in many countries (as mentioned in the Introduction). The critical role of the primitive streak/organizer in individuation is the reason why now again a broad discussion has been initiated about the use of human embryos in research, and on the production and use of human embryoids, since the technological advances let it appear quite probable that human embryoids will soon be able to reach a complexity beyond the primitive streak stage that may approach viability. Primitive streak/organizer formation is not the only aspect of concern: embryoids (not only mouse but also human) are being proposed by some to be attractive models for the study of the biology of germ line cell formation [94], an aspect that is usually not covered by ongoing discussions on informed consent to be obtained from embryo and stem cell donors [35], but certainly has to be included.
If an in vitro system allows for the production of large numbers of stem cell-derived embryoids, as in the experiments described by Kime et al. [71], research along these lines may of course be facilitated. In my opinion, such experiments, in particular if involving EPSCs and leading to the formation of blastocyst-like or gastruloid constructs, should, however, not be extended to human stem cells, for ethical reasons, since what is initiated here in stem cell colonies are, on a large scale, self-organization processes which can be enabled to continue up to basic body plan (primitive streak etc.) stages, i.e., starting individuation. I thus urge us to critically reconsider the ethical implications of producing and of using human embryoid constructs that can gain organismic wholeness (for a modern discussion of the organismicity aspect in developmental biology, I recomment to read [96]).
Would there be alternatives for the use of human stem cells, avoiding the ethical dilemma but allowing basic research on human embryoids to proceed? Obviously, in order to come as close as possible to the human system, non-human primate PPSCs may be used instead [93,95]. Specifically, we need to pay attention to questions like, under which conditions a group of stem cells may start the way to autonomy in the sense of gaining independence of pattern formation from outside signals, how this specific state of developmental autonomy can be detected, and how the process can be controlled. These questions open very important aspects to be urgently included in the ongoing discussions about new legal regulations concerning research on human stem cell-derived embryoids, since we are talking here about the biological basis of individuation. The gaining of developmental autonomy should be considered a quantum leap with regard to the dignity to be ascribed to a colony of stem cells, moving it into the same ethical category as an embryo of that stage. These aspects have, however, not yet been addressed in the recently published ISSCR Guidelines [99,100].
The article is a shortened version of a review previously published in Cells (Denker, H.-W. Autonomy in the Development of Stem Cell-Derived Embryoids: Sprouting Blastocyst-Like Cysts, and Ethical Implications. Cells 2021, 10, 1461; 10.3390/cells10061461)
Keywords
stem cells, embryoids, blastoids, gastruloids, blastocyst, expanded potential stem cells, development, morphogenesis, self-organization, ethics
© Text is available under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)
This entry is adapted from the peer-reviewed paper 10.3390/cells10061461