2.1. Reverse Transcription Quantitative PCR (RT-qPCR) Method
Detection of the SARS-CoV-2 viral genome, consisting of single-stranded RNA, is effectively done by reverse transcription quantitative polymerase chain reaction (RT-qPCR), which is the gold standard technique widely used in molecular diagnostics [
65,
66]. There are several practical considerations when performing diagnostic assays using RT-qPCR.
(1) Sample quality: RT-qPCR tests are presently being used for the identification of SARS-CoV-2 in clinical specimens such as upper respiratory tract specimens (saliva, oropharyngeal swab-OPS, nasopharyngeal swab-NPS, nasal swabs), lower respiratory specimens (sputum, bronchoalveolar lavage-BAL, endotracheal aspirate-ET, fibrobronchoscope brush biopsy-FBB), blood (serum, plasma), urine, feces, rectal/anal swabs, stool, and corneal secretion [
67,
68]. To check the sample quality of clinical specimens from different origins, an RNA isolation procedure is required to obtain purified high-quality RNA from the samples, which then needs to be analyzed using chip-based capillary electrophoresis (such as the Agilent Bioanalyzer system), electrophoretic separation on a high-resolution agarose gel, and spectrophotometry [
69].
(2) Reference curve: Data processing can critically affect the analysis of RT-qPCR results [
70]. PCR data processing is based on standard curves or on PCR efficiency assessments [
70]. Standard curves are used to assess RT-qPCR efficiency without theoretical and practical problems [
70]. The estimation of RT-qPCR efficiency using standard curves usually involves the serial dilution of a concentrated stock solution, after which standard samples are analyzed through RT-qPCR by measuring the quantification cycle (Cq) using standard procedures [
70]. The most widely used Cq value is the threshold cycle (Ct), the cycle at which the expression of a target gene first exceeds a calculated fluorescence threshold level [
71]. For example, to detect low amounts of SARS-CoV-2 RNA, a series of diluted RNA templates are used to determine the Ct value, which can provide a standard curve for evaluating the reaction efficiency [
72]. However, the Ct value itself cannot be directly explained as viral load without a standard curve using reference materials [
73]. When interpreting the results of SARS-CoV-2 RT-qPCR, the validity of the standard curve should be proved using reference materials with accurate viral copy numbers to interpret Ct values as viral loads [
73].
(3) Viral load: The success of virus isolation depends on the viral load [
74]. Viral loads in sputum samples and throat swabs are high when obtained within seven days after initial symptoms are observed, ranging from 10
4 to 10
7 copies per mL. This pattern is broken as low quantity of virus are obtained from samples taken after day 8 [
75]. In general, sputum samples show higher viral loads than throat swab samples, whereas low viral RNA is detected in urine or stool samples [
75]. The two main factors that influence the quantitative measurement of viral roads are Cq values that are repeatable with acceptable uncertainty and a reliable means of converting from the Ct value to viral load [
76,
77,
78]. For molecular diagnostic assays, a limit of detection (LoD) and a limit of quantification (LoQ) are also considered the lowest concentrations of target RNA that can be detected by RT-qPCR [
79].
(4) Sampling methods: RT-qPCR tests for SARS-CoV-2 have shown a high variation of false-negative rates (FNR) and false-positive rates (FPR) [
80,
81]. Numerous methods have been developed with the goal of improving the sensitivity, safety, and rapidity of COVID-19 tests by RT-qPCR. For example, one group tested the efficiency and sensitivity of SARS-CoV-2 detection of clinical specimens collected directly in nucleic acid stabilization and lysis buffer (NSLB), a mixture of lysis buffer and RNA preservative, instead of a viral transport medium (VTM), thus inactivating the virus immediately after sampling [
82].
(5) Sample source: To improve the expandability of SARS-CoV-2 testing, several sampling approaches have been developed including nasal, pooled nasal, and throat (oropharyngeal) swabs as well as saliva. Different clinical sampling methods affect the diagnostic performance of SARS-CoV-2 infection tests by RT-qPCR including sensitivity and specificity, and thus should be carefully considered [
83,
84,
85,
86]. The combined swab is largely recommended as a more appropriate specimen for diagnosis by RT-qPCR [
87,
88,
89].
(6) Sensitivity: The conserved regions, ORF 1ab (RNA-dependent RNA polymerase, RdRp), envelope (E), and nucleocapsid (N) genes of SARS-CoV-2, are usually selected as the standard target genes for primer and probe design [
90,
91]. However, initial reports of SARS-CoV-2 and other coronavirus sequences gave rise to an incorrect degenerate base that did not align with the SARS-CoV-2 RNA sequence found, and there were reports regarding the decreased sensitivity of using RdRp as a target gene for RT-qPCR assays [
90,
92]. As the pandemic continues, many laboratories around the world rely on routine diagnostic primers and probes. Thus, proper assays can increase the sensitivity of SARS-CoV-2 detection and help prevent the further spread of the virus [
92,
93,
94,
95].
(7) Pooling technologies: The pooling of multiple swab samples before RNA isolation and RT-qPCR analysis has been proposed as a promising solution to reduce costs and time as well as elevate the throughput of SARS-CoV-2 tests for large-scale testing as in the case of schools [
96,
97,
98,
99]. For example, batch testing of over 100,000 hospital-collected nasopharyngeal swab samples from patients alleviated three quarters of testing reactions with a minor reduction in sensitivity, indicating the effectiveness of the pooling approach in the field [
100,
101]. Current studies suggest that the pooling of individual samples before testing should be considered to increase the reliability of SARS-CoV-2 testing throughput.
Once all practical considerations have been evaluated, there are two ways that RT-qPCR can be performed. The two-step RT-qPCR method is required to convert RNA into complementary DNA (cDNA) [
102]. On the other hand, the one-step RT-qPCR method combines reverse transcription and PCR in a single tube and uses reverse transcriptase as well as a DNA polymerase [
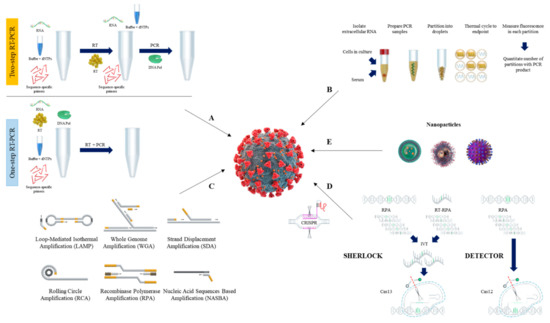
103]. The schematic procedure of RT-qPCR is shown in A.
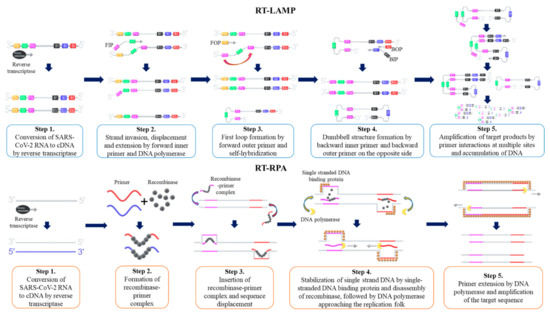
Figure 1. Overview of nucleic acid testing for SARS-CoV-2. The schematic procedure of RT-qPCR (A), and dPCR (B). Current isothermal amplification methods (C), CRISPR detection systems (D), and nanoparticles (E) are also shown.
A critical need for rapid and accurate diagnostic methods has emerged in the clinic and public health organizations. Several PCR-based assays have been developed and are currently being used in clinical, research, and public health laboratories [
104,
105,
106]. However, it is not clear which PCR condition they should adopt or whether the data are comparable. In response to the growing need and the lack of publicly available information, several research groups have optimized real-time PCR-based primer sets, protocols, and PCR conditions [
107,
108].
Independent evaluations of the designed primer–probe sets used in SARS-CoV-2 RT–qPCR detection assays are necessary to compare and select appropriate assays [
90,
109]. Additionally, several studies have utilized serum and stool specimens for the RT-qPCR-based detection method [
110,
111,
112,
113].