Prebiotic reactions describe how biomolecules such as ribose, was synthesized and protected from degradation, nucleobases generated from HCN oligomers and other derivatives including prebiotic RNA (preRNA) were formed at the given circumstances. Prebiotic conditions remained a subject of debate, but a reasonable aspect to consider is that tetrahedral structure shaping biomolecules consisted almost exclusively of elements belonging to the CHNOPS group in the periodic table, where capital letters correspond to the elements of carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur. Intermediary steps in the formose reaction network contain aldol condensation, aldose-ketose isomerizations, producing C3 - C6 carbohydrates including pentoses. The generation of genetic material could have developed from the formose reaction to ribose and further to non-genetic prebiotic RNA (preRNA) [1-9]. Consecutive reactions of this pathway are not known. Those known abiotic chemical reactions were selected that could have resulted in preRNA and genetic RNA (genRNA).
Processes generating primitive life on Earth consisted of three successive stages:
i) abiotic reactions generating organic molecules,
ii) formation of molecular aggregates showing primitive metabolism,
iii) development of primitive cells and organisms resembling those that exist today. Only cellular life is known that adapted in every aspect to conditions that exist only on Earth. Here only the 1st stage, the abiotic phase is dealt with.
Prebiotic conditions remained subjects of debate ranging from a strong reductive to a strong oxidative climate. Our theory is based on a mildly reductive atmosphere that could have existed at the beginning of life some 4.3 -4.5 billion years ago, much before the Great Oxidation Event that took place much later 2.3 - 2.5 billion years ago. During the abiotic synthesis of organic molecules, ribose was selected as the precursor to life.
.
- formose reaction system
- formation of carbohydrates
- fitting aldopentoses
- selection of ribose
- preRNA
- genRNA
1. Prebiotic Synthesis of Ribose
The oligomerization of formaldehyde is the traditional way to carbohydrate synthesis through the formose reaction resulting in a mixture of sugars of aldoses and ketoses of different chain lengths from trioses to hexoses [1][2][3][4]. The formose reaction is regarded as an autocatalytic self-condensation cycle [5] being autocatalytic in the formation of C2 and C3 sugars of glycolaldehyde and glyceraldehydes due to retro aldol reactions, that follow the initial synthesis from formaldehyde and, respectively glyceraldehyde, thus enhancing the stoichiometry of their formation. The formose reaction starts with the dimerization of formaldehyde followed by C2 to C6 saccharide formation. The origin of life is traced back to pentoses generated from mixtures of formaldehyde (C1), glyceraldehyde (C2), glyceraldehyde (C3), dihydroxyacetone (C3), aldoses (C2–C6), and ketoses (C2–C4) as well as minerals derived from boric acid (B(OH)3)). The formose reaction creates a mixture of sugars with varying sizes and shapes, of which ribose makes up less than 1%. Albert Eschenmoser’s group could significantly increase the yield of pentoses from the formose reaction by starting with formaldehyde and glycerolphosphate, which delivered up to 23% racemic ribose-2,4-diphosphate [6]. Thus Eschenmoser has proposed an alternative “glyoxylate scenario”, where glyoxylate [7] and its formal dimer, dihydroxyfumarate, were intended to be the central starting materials of a chemical constitution of primordial metabolism. These compounds served as sources for the formation of biogenic molecules such as sugars, including ribose. The selective formation of ketoses in the “glyoxylate scenario”, stands in stark contrast to the formose reaction, where a complex mixture of linear and branched aldoses and ketoses are produced and could constitute a pathway for the formation of carbohydrates. The two views of ribose formation advocate opposite sides of a research controversy. The reason why the formose reaction is favoured is given below.
Although, in the formose reaction at lower temperatures hexoses were synthesized in negligible yields but at a higher temperature (~200 °C) among pentoses ribose and ketoses resulted in a much higher proportion [4][8]. The missing selectivity for specific monosaccharide formation was one of the major problems of the formose reaction in the context of the origin of life [3][9][10]. The formose reaction encapsulated inside vesicles produced pentoses in 65% yield, which is much higher than the synthesis of C-5-monosaccharides under conventional conditions [11]. The same authors described that the system inside the vesicles as lipid-bound protometabolic units synthesizes complex carbohydrates and represents a fascinating example of an artificial cell capable of communication with natural cells [15]. Since ribose can be further transformed into flexible nucleotides where the intramolecular free rotation of functional groups is allowed and nucleotides can be polymerized to a genetic molecule [12]. The selective reactions favor the formose origin of ribose. Figure 1 shows ribose synthesis through the formose reaction.
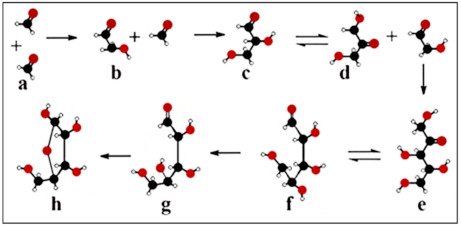
Figure 1. β-d-ribose synthesis in the formose reaction. Two formaldehyde molecules (a) condensed to glycolaldehyde (b). The subsequent aldol condensation is forming glyceraldehyde (c), which undergoes a ketose-aldose isomerization (d). Glyceraldehyde reacts with glycolaldehyde to bring about pentulose (e). Selective isomerization takes place between pentulose (e) and d-ribose (f), favouring the nucleophilic addition reaction of d-ribose (g) and ring formation to β-d-ribose (h).
It deserves to be mentioned in this context that the formose reaction does not deliver d-ribose enantioselectively, but only racemic ribose. d-ribose depicted here (for clarity purposes) is the natural enantiomer of abiotic origin.
In nucleotides containing arabinose, xylose, or lyxose, the C2-OH or the C3-OH and the C5-OH groups are in the opposite orientation of the furanose ring substituents causing steric interference caused by the vicinity of the large base and/or the C5-OH group (Figure 1e). After ring formation in β-d-ribose all substituents are as far from each other as possible. The juxtaposed position of substituents (Figure 1h) provides free rotation of the substituents of β-d-ribose and high stability to the molecule. The conformers originating from sugar pucker folding as well as pentose configurations indicated that the selection of ribose was not a random process, but the only possible solution. β-d-ribose perfectly fits into functional nucleic acids, whereas other pentoses cause steric hindrances [13]. Thus for the formation of a flexible RNA chain, only β-d-ribose turned out to be suitable (Figure 2).
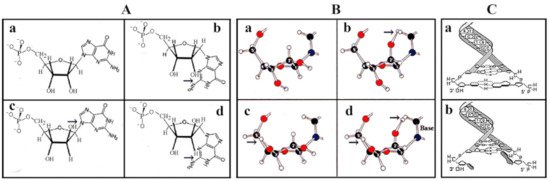
Figure 2. Selection of β-d-ribose as the best fitting pentose in the nucleotide structure. Irrespective of the formose or glyoxylate origin of ribose and the improved production of pentoses did not answer the question of why pentoses, particularly ribose, were selected as the exclusive sugar component of the genetic material. Molecular modeling revealed that free rotation of functional groups (OH, phosphate, nucleobase) of ribose inside nucleotides takes place only in β-d-ribose. (A/a), confirmed in (B/a). The free rotation of functional groups of the beta anomer of arabinose is hindered by its 2-OH moiety (A/c). As far as the alpha anomers of pentoses are concerned, the bulky bases are too close to the sugar and would not allow its free rotation (A/b,d). (B) panels confirm that free rotation of the substituents is permitted only in β-d-ribose (B/a) but not in arabinose, xylose, or lyxose (B/b,c,d). The free movement of functional groups of ribose turned out to be essential to provide maximal flexibility of RNA and to secure the stability and base pairing of deoxyribose in double-stranded DNA (C). Double helix formation is possible only in β-d-deoxyribonucleotides (C/a) but not between the α-d-deoxyribonucleotide pairs where the nucleobases are not perpendicular to the axis of the helix and will prevent hydrogen bonding between bases (C/b). With permission [12].
It should be also noted in this context that modeling with d-pentoses could also be modeled with l-pentoses and would yield identical energies and shapes, if not enantiomeric, provided that no mixture of d and l enantiomers were permitted [13]. However, the mirror image l-ribose is not found in nature [14].
2. Prebiotic Synthesis of Purine and Pyrimidine Nucleobases
The prebiotic synthesis of purine nucleobases under primitive Earth conditions [24] and the prebiotic pyrimidine synthesis started from cyanoacethylene (HC≡CCN) and cyanate (NCO−) [15]. Cyanyl (NCO) does not exist except under extreme vacuum conditions or in the cosmos, since it is a radical. The neutral, moderately stable compound is isocyanic acid (HNCO) . Isocyanic acid forms upon strong heating of urea that eliminates ammonia to give isocyanic acid as a highly reactive liquid or gas. Ammonia and isocyanic acid can produce a quite volatile salt, ammonium cyanate. Cyanate as a sodium, potassium (or other metal cation) salt is more stable, but much less reactive, too. The chemical stability of the nitrogen-containing heterocyclic nucleobases suggests that they were among the first stable prebiotic molecules that have been formed by using HCN as the primary precursor. Less attention is paid here to the synthesis of nucleobases as it does not belong to the major focus of this study.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22083857
References
- Boutlerow, A. Formation Synthétique d’une Substance Sucrée. CR Acad. Sci. 1861, 53, 145–147.
- Zafar, I.; Senad, N. The formose reaction: A tool to produce synthetic carbohydrates within a regenerative life support system. Curr. Org. Chem. 2012, 16, 769–788.
- Delidovich, I.V.; Simonov, A.N.; Taran, O.P.; Parmon, V.N. Catalytic Formation of Monosaccharides: From the Formose Reaction towards Selective Synthesis. ChemSusChem 2014, 7, 1833–1846. [CrossRef]Int. J. Mol. Sci. 2021, 22, 3857.
- Breslow, R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959, 1, 22–26.
- Huskey, W.P.; Epstein, I.R.J. Auto-catalysis and apparent bistability in the formose reaction. Am. Chem. Soc. 1989, 111, 3157–3163.
- Müller, D.; Pitsch, S.; Kittaka, A.; Wagner, E.; Wintner, C.E.; Eschenmoser, A.; Ohlofjgewidmet, G. Chemie von a-Aminonitrilen. Aldomerisierung von Glycolaldehyd-phosphat zu racemischen Hexose-2,4,6-triphosphaten und (in Gegenwart von Formaldehyd) racemischen Pentose-2,4-diphosphaten: Rac-Allose-2,4,6-triphosphat und rac-Ribose-2,4-diphosphat sind die R. Helv. Chim. Acta 1990, 73, 1410–1468.
- Eschenmoser, A. The search for the chemistry of life’s origin. Tetrahedron 2007, 63, 12821–12844.
- Benner, S.A.; Bell, E.A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.; Mojzsis, S.J.; Omran, A.; Pasek, M.A.; Trail, D. When Did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem 2020, 2, 1900035.
- Kopetzky, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794.
- Lamour, S.; Pallmann, S.; Haas, M.; Trapp, O. Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration. Life 2019, 9, 52.
- Gardner, P.M.; Winzer, K.; Davis, B.G. Sugar synthesis in a protocellular model leads to a cell signalling response in bacteria. Nat. Chem. 2009, 1, 377–383.
- Banfalvi, G. Ribose Selected as Precursor to Life. DNA Cell Biol. 2020, 39, 177–186.
- Okano, K. Synthesis and pharmaceutical application of L-ribose. Tetrahedron 2009, 65, 1937–1949.
- Banfalvi, G. Why ribose was selected as the sugar component of nucleic acids. DNA Cell Biol. 2006, 25, 189–196.
- Ferris, J.P.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis. 3. synthesis of pyrimidines from cyanoacetylene and cyanate. J. Mol. Biol. 1968, 33, 693–704.
