The human gut microbiota has been defined as the entire collection of microbes (bacteria, archaea, eukarya, and viruses) living as a complex ecosystem in our gastrointestinal tract, coevolved with humankind.
Diet have a fundamental role in determining gut microbiota composition and diversity.
Recent studies have also suggested the possible influence of physical activity (PA) on gut microbiota composition.
The aim of this systematic review is to better understand whether and how PA can influence human gut microbiota composition independently of diet. To this aim, controlled studies exploring the relationship between PA and human microbiome in healthy individuals were evaluated. The findings of these studies were examined in light of the possible confounding effect of dietary factors.
- physical activity
- diet
- microbiota
- human
- gut
- healthy
- biodiversity
1. Introduction
The composition and diversity of intestinal microbiota have been associated with several chronic diseases, including colorectal cancer, metabolic, autoimmune, and allergic diseases, and neurological disorders[1][2][3][4][5][6][7][8][9][10][11][12][13]. Gut microbes can affect the homeostasis of the host by producing vitamins, amino acids, and short-chain fatty acids (SCFA) starting from food components[14]. Therefore, by providing substrates for microbial metabolism, the diet has a fundamental role in determining gut microbiota composition and diversity[15][16]. Recent studies have also suggested the possible influence of physical activity (PA) on gut microbiota composition. Evidence suggests that some microbial phyla and genera whose abundance was found to be exercise-related, especially SCFA producers, may have a role in maintaining intestinal epithelial homeostasis and increasing mucus thickness, in improving host metabolic immune status, and in modulating the gut–brain axis, reducing, among other things, neuroinflammation and mental fatigue. However, the majority of the available studies focused on this issue are based on animal models or performed among non-healthy individuals. In addition, the studies performed in humans have heterogeneous designs and they consider different forms, doses, and duration of exercise, which did not allow to draw clear conclusions[17][18][19][20][21][22][23]. Furthermore, the interaction between PA and diet composition and their respective influence on gut microbiota are not always characterized[24][25]. This systematic review is aimed to better understand whether and how PA can influence human gut microbiota composition independently of diet.
2. Diet-independent relationship between PA and Gut Microbiota Composition
Recent studies have suggested that higher levels of PA and cardiorespiratory fitness are associated with higher microbial diversity in the gut and with the abundance of some phyla and certain SCFA producers in humans[19][20]. Previous reviews have tried to address this item, but they did not reach clear conclusions due to the paucity and the heterogeneity of the available studies on humans[21][22][23][24][25][26]. Since the designs of the studies, the populations examined, the types of PA considered, or the assessment methods are different in the literature, it is difficult to draw definite statements in this field. In their recent review, Tzemah Shahar et al. tried to characterize the role of PA in humans by analyzing exclusively studies reporting PA intervention with a duration of at least 5 weeks[26]. Aya et al., instead, included cross-sectional and longitudinal studies[25]. However, both reviews did not evaluate these studies in the light of how the relationship between PA and diet was considered.
Since the gut microbiota may be influenced by several factors, such as diet or disease, it is important to characterize the possible role of PA excluding the effects of potential confounders. Therefore, while planning this review, we have tried to choose eligibility criteria that could have allowed us to obtain a selection of comparable studies. To this aim, we considered only controlled studies on healthy humans and evaluated their findings with regard to the diet, assumed as the main possible confounder. Furthermore, in the analysis of the results, we separated those obtained from athlete groups and those coming from the general population to detect possible differences related to sports practice, active lifestyle, and sedentarism [27][28][29][30][31][32][33][34][35][36].
The selected studies showed heterogeneous results regarding microbiome variability and composition, so as for diet-related outcomes. This is probably due to the differences among the studies, which employed different measures and have been conducted on samples from different geographical areas, practicing different sports or levels of exercise, and with different ages. As for microbial variability, six out of ten studies found higher values in active people[27][29][30][31][32]. In addition, the study of Bressa et al. reported an inverse association between sedentary parameters and microbiota richness, suggesting that the pattern of exercise, such as breaks in sedentary time, avoiding long periods of inactivity during the daily routine, may induce changes in gut microbiota composition[28]. However, two of these[30][31] did not report differences when using other variability indexes, and another found lower dissimilarity in athletes with respect to controls [29]. These different results may be attributed to the different measures adopted to evaluate microbiota variability and are consistent with those of Tzemah Shahar et al.[26]. Interestingly, the studies which showed higher variability were performed in young adult groups [27][29][30][31][32]. The study by Taniguchi et al., which did not register different levels of variability, involved older adults[36]. It is possible that the different age classes have played a role in determining these results. In fact, compared with young adults, the elderly have different digestive physiology, characterized by a reduction in transit and production of digestive secretions, which could explain the changes in the fecal microbiota associated with advancing age[37]. In contrast with this, Jang et al. did not find significant microbiota diversity between young athletes and healthy controls. However, as the authors stated, the inadequate intake of carbohydrates and dietary fiber associated with a high protein diet observed in the athletes might have counteracted the beneficial effects of exercise on gut microbiota diversity. This unbalanced diet may be the cause of the inconsistency between these results and those of Clarke et al. and Cronin et al., who reported a higher microbial diversity in rugby athlete/exercise groups in relation to protein assumption [29][30]. Even analyzing a sample of rugby players, Barton et al. confirmed the enhancement of microbial diversity in professional athletes who associate extreme PA and dietary adaptations, such as increased protein intake, in comparison with sedentary individuals. Athletes showed also increased metabolic pathways and fecal metabolite production than controls[27].
As reported by other authors[24][25], several differences between athlete/active and inactive people were instead reported at phylum and genus level, even by studies that did not detect significant variations in general variability measures. The majority of the studies found a higher abundance of the phylum Firmicutes or correspondent genera[27][28][29][30][31][32][33][34][35] and a lower prevalence of Bacteroidetes or related genera[28][29][31][32][33] in more active people. Contrarily to these results, a lower prevalence of C. difficile was detected in the exercise group by Taniguchi et al.[36], while a higher prevalence of the Prevotella genus was found in rugby players by Clarke et al.[29]. The Firmicutes/Bacteroidetes ratio has been found to be associated with several factors that can influence their balance in the gut. Age, gender, therapies, diet may in fact favor one of these phyla, leading to dysbiosis which can, in turn, allow the development of diseases. In particular, the increased amount of Firmicutes with respect to Bacteroidetes has been related to the pathophysiology of intestinal, metabolic, and Central Nervous System-related disorders[38][39][40]. However, since the increase of some Firmicutes genera cannot be necessarily negative for the host, the findings of this review do not indicate necessarily that exercise may be detrimental for gut microbiome composition and human health. In fact, Firmicutes genera such as Ruminococcaceae or Fecalibacteria, which were reported to be higher among active participants in the studies by Clarke, Jung, and Han, have been shown to be beneficial for health and they were associated with healthy status and lifestyle[29][32][33]. At the same time, the genus Megasphaera, which was reported to be lower in active individuals by Gallè et al., and the genus Bacteroides, whose decrease was found by Clarke and Jang, were associated with a disease status[31][29][33]. Interestingly, among Verrucomicrobia, an increase of the genus Akkermansia was found among active people in three studies[27][28][29]. The role of this intestinal symbiont in host health has been widely shown[39]. In particular, the levels of A. muciniphila have been demonstrated to be negatively correlated with some diseases, included inflammatory bowel disease, obesity, and diabetes[40]; its activity in increasing intestinal mucus thickness, gut barrier, and immune signaling functions, and its role as SCFAs producer made this species a promising candidate for next-generation probiotics. Other findings at genus level were study-specific and account for differences among athletes practicing different types of sport.
In conclusion, the results of this systematic review indicate that PA can increase the abundance of health-promoting bacteria in the intestinal microbiota, hindering some negative genera. In particular, a higher variability and abundance of Firmicutes was reported in the majority of the studies comparing athletes and sedentary people, while these findings were less robust in the studies performed in the general population. This could be related to the different volume of exercise existing between athletes and non-athletes and may suggest the importance of the volume of PA in determining gut microbiota composition. However, it should be noted that the practice of sport can be associated with specific food/nutrient intakes, which can favor specific microbial populations[21][24][25]. Therefore, it may be difficult to disentangle the effects of PA/exercise and diet. In our analysis, only four cross-sectional investigations adjusted their results for diet (Figure 1)[31][34][35][28].
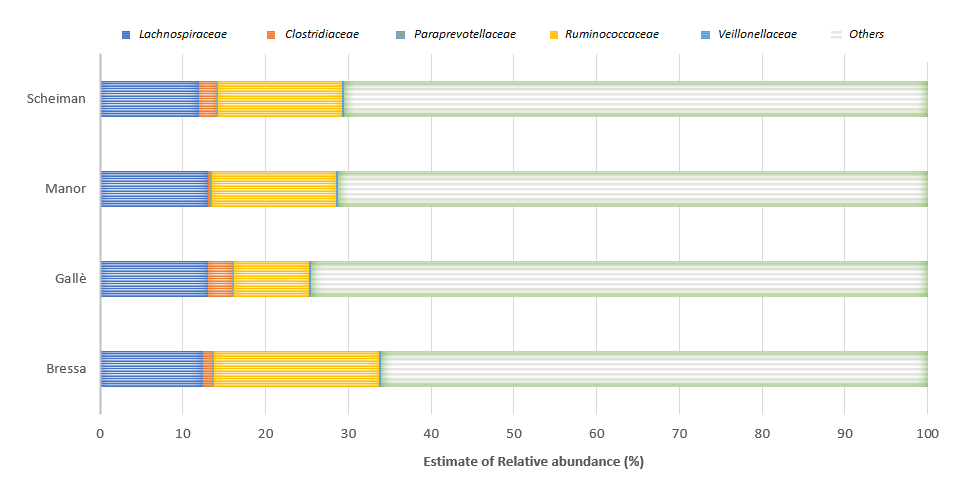
Figure
Two of these studies refer to the lack of significant correlations after controlling for confounders, while Manor et al. confirmed a significant association between PA and microbiome diversity after adjusting for dietary factors [38]. The study by Scheiman et al. found a diet-independent increase of the genus Veillonella, suggesting the possibility that systemic lactate resulting from muscle activity during exercise may enter the gastrointestinal lumen and become metabolized by this genus, providing a selective advantage for the gut colonization by lactate-metabolizing organisms[35]. Interestingly, the relationship between PA levels and Veillonella abundance in the gut was reported also by Manor et al.[34]. Moreover, it should be noted that these four studies highlighted similar findings regarding the abundance of bacterial families (Lachnospiraceae, Paraprevotellaceae, Ruminococcaceae, and Veillonellaceae) that are involved in several functions and different pathways, including metabolic, protective, structural, and histological functions. Lachnospiraceae include genus as Coprococcus, a butyrate-producing genus, that promotes some exercise-related health effects. Moreover, Lachnospira species are known to produce anti-inflammatory short-acid butyrate[37]. Furthermore, the families Lachnospiraceae and Ruminococcaceae can be anticorrelated in gut microbiota because they overlap as an ecological niche. Indeed, these families can respond to similar diets, such as high fiber or probiotics[41]. However, both can be involved in fiber degradation and butyrate production[41][32]. Clostridiaceae are associated with an increase in fecal butyrate production amongst physically fit participants and involved in these pathways [42][43]. However, this bacterial family can be influenced by the intake of a high-fat diet[44]. Veillonellaceae are involved in lactate metabolism and contribute to the dihydroxylation of bile acids[45]. Indeed, some species metabolize lactate into SCFAs acetate and propionate via the methyl-malonyl-CoA pathway[35][45].
However, apart from these few specific findings, given the variability in populations examined and diet assessment tools employed in these studies, their results cannot be collated, and no robust conclusions can be expressed regarding the independent effects of PA/exercise on the gut microbiota of healthy humans.
This review was an attempt to summarize the evidence about gut microbial diversity and abundance related to PA trying to separate the main available evidence from diet contribution, which differentiates our analysis from previous reviews performed on this item. However, it shows some limitations. First of all, it should be considered that the majority of the selected studies did not collect lifestyle information through objective measurement tools, such as accelerometers for PA/exercise or photographic monitoring of dietary intake. Assessing these variables through self-reporting may lead to inaccurate results. Furthermore, separating the effects of diet and PA is difficult to perform, since PA itself can favor the adoption of specific dietary patterns.
More randomized controlled studies, analyzing wider samples, and controlling for potential confounders are needed in this field to disentangle this question. In particular, it should be considered that the practice of sport is often associated with specific and sometimes extreme dietary patterns in professional athletes, so as regular exercise in amateurs is often accompanied by healthier dietary habits[21][22][23][27][29][34]. In addition, the gut microbiome has been shown to mediate the effect of both diet and exercise, making it relevant to the athletes’ health and performance[46]. Therefore, in light of optimizing microbiota functionality for both athletes and the general population through the design of adequate exercise and dietary programs, the components of the exercise and diet–microbiome paradigm should be further explored.
(References would be added automatically after the entry is online)
This entry is adapted from the peer-reviewed paper 10.3390/nu13061890
References
- Shreiner, A. B., Kao, J. Y., Young, V. B. The gut microbiome in health and in disease. Curr Opin Gastr, 2015, 31(1), 69–75.
- Qin, J., Li, R., Raes, J., et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature, 2010, 6(7285), 59-65.
- Eckburg, P.B., Bik, E.M., Bernstein, C.N., et al. Microbiology: diversity of the human intestinal microbial flora. Science, 2005; 308 (5728), 1635–1638.
- Backhed, F. Host-Bacterial Mutualism in the Human Intestine. Science, 2005, 307(5717), 1915-1920.
- Grenham, S., Clarke, G., Cryan, J.F., Dinan, T.G. Braingut-microbe communication in health and disease. Frontiers in Physiology, 2011, 2(94), 1-15.
- Prakash, S., Rodes, L., Coussa-Charley, M., Tomaro-Duchesneau, C. Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biol Targets Ther., 2011, 5-71.
- de Vos, W.M., de Vos, E.A. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev., 2012, 70, S45-S56.
- Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D., Hirschfield, G. M., Hold, G., Quraishi, M. N., Kinross, J., Smidt, H., Tuohy, K. M., Thomas, L. V., Zoetendal, E. G., Hart, A. The gut microbiota and host health: a new clinical frontier. Gut, 2016, 65(2), 330–339.
- Toor, D., Wsson, M. K., Kumar, P., Karthikeyan, G., Kaushik, N. K., Goel, C., Singh, S., Kumar, A., Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int J Mol Sci, 2019, 20(10), 2432.
- Nie, P., Li, Z., Wang, Y., Zhang, Y., Zhao, M., Luo, J., Du, S., Deng, Z., Chen, J., Wang, Y., Chen, S., Wang, L. Gut micro-biome interventions in human health and diseases. Med Res Rev, 2019, 39(6), 2286-2313.
- Belkaid, Y., Hand, T.W. Role of the microbiota in immunity and inflammation. Cell. 2014, 157(1), 121-141.
- Fulbright, L.E., Ellermann, M., Arthur, J.C. The microbiome and the hallmarks of cancer. PLoS Pathog 2017, 13(9), e1006480.
- Orsini, M., Di Liddo, R., Valeriani, F., Mancin, M., D'Incà, R., Castagnetti, A., Aceti, A., Parnigotto, P. P., Romano Spica, V., Michetti, F. In Silico Evaluation of Putative S100B Interacting Proteins in Healthy and IBD Gut Microbiota. Cells., 2020, 9(7), 1697.
- Roager, H.M., Dragsted, L.O. Diet‐derived microbial metabolites in health and disease. Nutr Bull, 2019, 44(3), 216-227.
- Rinninella, E., Cintoni, M., Raoul, P., Lopetuso, L. R., Scaldaferri, F., Pulcini, G., Miggiano, G., Gasbarrini, A., & Mele, M. C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients, 2019, 11(10), 2393.
- Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., Almeida, M., Quinquis, B., Levenez, F., Galleron, N., Gougis, S., Rizkalla, S., Batto, J. M., Renault, P., ANR MicroObes consortium, Doré, J., Zucker, J. D., Clément, K., & Ehrlich, S. D. Dietary intervention impact on gut microbial gene richness. Nature, 2013, 500, 585-588.
- Monda, V., Villano, I., Messina, A., Valenzano, A., Esposito, T., Moscatelli, F., Viggiano, A., Cibelli, G., Chieffi, S., Monda, M., & Messina, G. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev, 2017, 3831972.
- Mailing, L.J., Allen, J.M., Buford, T.W., Fields, C.J., Woods, J.A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev, 2019, 47(2), 75-85.
- Gallè, F., Valeriani, F., Cattaruzza, M. S., Ubaldi, F., Romano Spica, V., Liguori, G., WDPP, Working Group on Doping Prevention Project, & GSMS-SItI, Working Group on Movement Sciences for Health, Italian Society of Hygiene, Pre-ventive Medicine and Public Health. Exploring the association between physical activity and gut microbiota composi-tion: a review of current evidence. Ann Ig., 2019, 31(6), 582-589.
- Cerdá, B., Pérez, M., Pérez-Santiago, J.D., Tornero-Aguilera, J.F., González-Soltero, R., Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol., 2016, 7, 51.
- Mitchell, C. M., Davy, B. M., Hulver, M. W., Neilson, A. P., Bennett, B. J., Davy, K. P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med Sci Sports Exerc., 2019, 51(1), 160–167.
- Warburton, D., Bredin, S. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017, 32(5), 541–556.
- Ortiz-Alvarez, L., Xu, H., & Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin Transl Gastroenterol., 2020, 11(2), e00126.
- Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016,13:43.
- Aya, V., Flórez, A., Perez, L., & Ramírez, J. D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PloS one, 2021,16(2), e0247039.
- Tzemah Shahar, R., Koren, O., Matarasso, S., Shochat, T., Magzal, F., & Agmon, M. Attributes of Physical Activity and Gut Microbiome in Adults: A Systematic Review. Int J Sports Med. 2020,41(12),801-814.
- Barton, W., Penney, N. C., Cronin, O., Garcia-Perez, I., Molloy, M. G., Holmes, E., Shanahan, F., Cotter, P. D., & O'Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut., 2018, 67(4), 625-633
- Bressa, C., Bailén-Andrino, M., Pérez-Santiago, J., González-Soltero, R., Pérez, M., Montalvo-Lominchar, M. G., Ma-té-Muñoz, J. L., Domínguez, R., Moreno, D., Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One, 2017, 12(2), e0171352.
- Clarke, S. F., Murphy, E. F., O'Sullivan, O., Lucey, A. J., Humphreys, M., Hogan, A., Hayes, P., O'Reilly, M., Jeffery, I. B., Wood-Martin, R., Kerins, D. M., Quigley, E., Ross, R. P., O'Toole, P. W., Molloy, M. G., Falvey, E., Shanahan, F., Cotter, P. D. Exercise and associated dietary extremes impact on gut microbial diversity. Gut, 2014, 63(12), 1913-1920.
- Cronin, O., Barton, W., Skuse, P., Penney, N. C., Garcia-Perez, I., Murphy, E. F., Woods, T., Nugent, H., Fanning, A., Melgar, S., Falvey, E. C., Holmes, E., Cotter, P. D., O'Sullivan, O., Molloy, M. G., & Shanahan, F. A Prospective Meta-genomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Mi-crobiome of Sedentary Adults. mSystems, 2018, 3(3), e00044-18.
- Gallè, F., Valeriani, F., Cattaruzza, M. S., Gianfranceschi, G., Liguori, R., Antinozzi, M., Mederer, B., Liguori, G., Romano Spica, V. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients., 2020, 12(7), 2164.
- Han, M., Yang, K., Yang, P., Zhong, C., Chen, C., Wang, S., Lu, Q., Ning, K. Stratification of athletes' gut microbiota: the multifaceted hubs associated with dietary factors, physical characteristics and performance. Gut Microbes., 2020, 12(1), 1-18.
- Jang, L. G., Choi, G., Kim, S. W., Kim, B. Y., Lee, S., Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J Int Soc Sports Nutr., 2019, 16(1), 21.
- Manor, O., Dai, C.L., Kornilov, S.A., Smith, B., Price, N.D., Lovejoy, J.C., Gibbons, S.M., Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun., 2020, 11(1), 5206.
- Scheiman, J., Luber, J. M., Chavkin, T. A., MacDonald, T., Tung, A., Pham, L. D., Wibowo, M. C., Wurth, R. C., Pun-thambaker, S., Tierney, B. T., Yang, Z., Hattab, M. W., Avila-Pacheco, J., Clish, C. B., Lessard, S., Church, G. M., Kostic, A. D. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate me-tabolism. Nat Med., 2019, 25(7), 1104-1109.
- Taniguchi, H., Tanisawa, K., Sun, X., Kubo, T., Hoshino, Y., Hosokawa, M., Takeyama, H., Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol Rep., 2018, 6(23), e13935.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms, 2019, 7, 14.
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930.
- Ribeiro, F.M.; Lopes, G.; da Cunha Nascimento, D.; Pires, L.; Mulder, A.P.; Franco, O.L.; Petriz, B. An overview of the level of dietary support in the gut microbiota at different stages of life: A systematic review. Clin. Nutr. ESPEN 2021, 42, 41–52.
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaruaes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol, 2009, 9, 123.
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in micro-biome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642.
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24.
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardi-orespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42.
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73.
