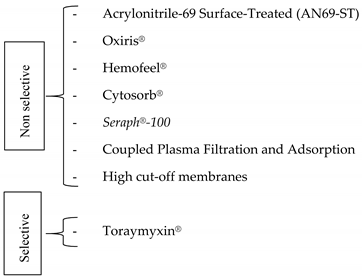
2.4. Filling the Gap of Immune Modulation in Sepsis
Immune modulation offers enticing perspectives of treatment for critically ill septic patients. However, the real application of this complementary treatment is still a matter of debate due to controversial results between laboratory and clinical trials. Sepsis is a clinical syndrome, which complex pathophysiology may be explained by the multifaced genetic (e.g., polymorphic inflammatory pathways) and epigenetic (e.g., comorbidities and clinical intervention applied) interplay that characterizes each single patient. Accordingly, a personalized approach to sepsis may address such a gap via the clinical application of biomarkers of single-cell transcriptomics [
68], big data analysis [
69], and machine-learning methods by specific models [
70], in order to identify specific patient populations that may benefit more from some specific immune modulating intervention and help the design of future clinical trials.
4. Conclusions
Immune modulation represents a complementary therapy for critically ill patients with sepsis. Among immune modulating strategies, EBPT appear safe and timely targeted compared with longer lasting pharmacological therapies. However, little evidence supports the efficacy of immune modulation in critically ill patients with sepsis. Accordingly, immune modulation remains a matter of debate and further research, carried out by evidence-based and personalized approaches, is warranted in order to improve the management of critically ill septic patients.
References
1. Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [CrossRef]
2. Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [CrossRef]
3. Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [CrossRef] [PubMed]
4. Cutuli, S.; De Pascale, G.; Antonelli, M. ’ση´ψις’ yesterday, sepsis nowadays: What’s changing? J. Thorac. Dis. 2017, 9, E166–E167. [CrossRef]
5. De Pascale, G.; Cutuli, S.; Pennisi, M.; Antonelli, M. The role of mannose-binding lectin in severe sepsis and septic shock. Mediat. Inflamm. 2013, 2013, 625803. [CrossRef] [PubMed]
6. Davenport, E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.S.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [CrossRef]
7. Cutuli, S.L.; Carelli, S.; De Pascale, G. The gut in critically ill patients: How unrecognized “7th organ dysfunction” feeds sepsis. Minerva Anestesiol. 2020, 86, 595–597. [CrossRef] Medicina 2021, 57, 552 9 of 11
8. Cutuli, S.L.; De Maio, F.; De Pascale, G.; Grieco, D.L.; Monzo, F.R.; Carelli, S.; Tanzarella, E.S.; Pintaudi, G.; Piervincenzi, E.; Cascarano, L.; et al. COVID-19 influences lung microbiota dynamics and favors the emergence of rare infectious diseases: A case report of Hafnia Alvei pneumonia. J. Crit. Care 2021, 64, 173–175. [CrossRef]
9. Hotchkiss, R.; Moldawer, L.; Opal, S.; Reinhart, K.; Turnbull, I.; Vincent, J. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [CrossRef]
10. Boomer, J.; Green, J.; Hotchkiss, R. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence 2014, 5, 45–56. [CrossRef] [PubMed]
11. Marshall, J.C.; Foster, D.M.; Vincent, J.; Cook, D.J.; Cohen, J.; Dellinger, R.P.; Opal, S.M.; Abraham, E.H.; Brett, S.J.; Smith, T.J.; et al. Diagnostic and Prognostic Implications of Endotoxemia in Critical Illness: Results of the MEDIC Study. J. Infect. Dis. 2004, 190, 527–534. [CrossRef] [PubMed]
12. Rubartelli, A.; Lotze, M.T. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007, 28, 429–436. [CrossRef] [PubMed]
13. Binnie, A.; Tsang, J.L.; Hu, P.; Carrasqueiro, G.; Castelo-Branco, P.; Dos Santos, C.C. Epigenetics of Sepsis. Crit. Care Med. 2020, 48, 745–756. [CrossRef] [PubMed]
14. Wolff, N.S.; Hugenholtz, F.; Wiersinga, W.J. The emerging role of the microbiota in the ICU. Crit. Care 2018, 22, 78. [CrossRef]
15. Kitsios, G.D.; Morowitz, M.J.; Dickson, R.P.; Huffnagle, G.B.; McVerry, B.J.; Morris, A. Dysbiosis in the intensive care unit: Microbiome science coming to the bedside. J. Crit. Care 2017, 38, 84–91. [CrossRef]
16. Yadav, H.; Cartin-Ceba, R. Balance between Hyperinflammation and Immunosuppression in Sepsis. Semin. Respir. Crit. Care Med. 2016, 37, 042–050. [CrossRef]
17. Vincent, J.; Moreno, R.; Takala, J.; Willatts, S.; Mendonça, A.D.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [CrossRef]
18. Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.-L. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001, 286, 1754–1758. [CrossRef]
19. Seymour, C.; Liu, V.; Iwashyna, T.; Brunkhorst, F.; Rea, T.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [CrossRef]
20. Mathias, B.; Szpila, B.E.; Moore, F.A.; Efron, P.A.; Moldawer, L.L. A Review of GM-CSF Therapy in Sepsis. Medicine 2015, 94, e2044. [CrossRef] [PubMed]
21. Döcke, W.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.-D.; Kox, W. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat. Med. 1997, 3, 678–681. [CrossRef]
22. Piguet, P.F.; Grau, G.E.; De Kossodo, S. Role of Granulocyte-Macrophage Colony-Stimulating Factor in Pulmonary Fibrosis Induced in Mice by Bleomycin. Exp. Lung Res. 1993, 19, 579–587. [CrossRef]
23. Serafini, P.; Carbley, R.; Noonan, K.A.; Tan, G.; Bronte, V.; Borrello, I. High-Dose Granulocyte-Macrophage Colony-Stimulating Factor-Producing Vaccines Impair the Immune Response through the Recruitment of Myeloid Suppressor Cells. Cancer Res. 2004, 64, 6337–6343. [CrossRef]
24. Shindo, Y.; Fuchs, A.G.; Davis, C.G.; Eitas, T.; Unsinger, J.; Burnham, C.-A.D.; Green, J.M.; Morre, M.; Bochicchio, G.V.; Hotchkiss, R.S. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J. Leukoc. Biol. 2017, 101, 543–554. [CrossRef] [PubMed]
25. Unsinger, J.; Burnham, C.-A.D.; McDonough, J.; Morre, M.; Prakash, P.S.; Caldwell, C.C.; Dunne, W.M.; Hotchkiss, R.S. Interleukin7 Ameliorates Immune Dysfunction and Improves Survival in a 2-Hit Model of Fungal Sepsis. J. Infect. Dis. 2012, 206, 606–616. [CrossRef]
26. Rittirsch, D.; Flierl, M.; Nadeau, B.; Day, D.; Huber-Lang, M.; Mackay, C.R.; Zetoune, F.S.; Gerard, N.P.; Cianflone, K.; Köhl, J.; et al. Functional roles for C5a receptors in sepsis. Nat. Med. 2008, 14, 551–557. [CrossRef] [PubMed]
27. Oncul, S.; Afshar-Kharghan, V. The interaction between the complement system and hemostatic factors. Curr. Opin. Hematol. 2020, 27, 341–352. [CrossRef]
28. Vincent, J.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.-B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients with Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA 2019, 321, 1993–2002. [CrossRef]
29. Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.-F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin Alfa (Activated) in Adults with Septic Shock. N. Engl. J. Med. 2012, 366, 2055–2064. [CrossRef] [PubMed]
30. Shankar-Hari, M.; Spencer, J.; Sewell, W.; Rowan, K.M.; Singer, M. Bench-to-bedside review: Immunoglobulin therapy for sepsis -biological plausibility from a critical care perspective. Crit. Care 2012, 16, 206–214. [CrossRef]
31. Alejandria, M.M.; Lansang MA, D.; Dans, L.F.; Mantaring, J.B., III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 2013, 2013, CD001090. [CrossRef]
32. Laupland, K.B.; Kirkpatrick, A.W.; Delaney, A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: A systematic review and meta-analysis. Crit. Care Med. 2007, 35, 2686–2692. [PubMed]
33. Peeters, B.; Langouche, L.; Berghe, G.V.D. Adrenocortical Stress Response during the Course of Critical Illness. Compr. Physiol. 2017, 8, 283–298. [CrossRef] Medicina 2021, 57, 552 10 of 11
34. Heming, N.; Sivanandamoorthy, S.; Meng, P.; Bounab, R.; Annane, D. Immune Effects of Corticosteroids in Sepsis. Front. Immunol. 2018, 9, 1736. [CrossRef]
35. Gibbison, B.; López-López, J.A.; Higgins, J.P.T.; Miller, T.; Angelini, G.D.; Lightman, S.L.; Annane, D. Corticosteroids in septic shock: A systematic review and network meta-analysis. Crit. Care 2017, 21, 1–8. [CrossRef]
36. Rygård, S.L.; Butler, E.; Granholm, A.; Møller, M.H.; Cohen, J.; Finfer, S.; Perner, A.; Myburgh, J.; Venkatesh, B.; Delaney, A. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018, 44, 1003–1016. [CrossRef]
37. Torres, A.; Sibila, O.; Ferrer, M.; Polverino, E.; Mendendez, R.; Mensa, J.; Gabarrus, A.; Sellares, J.; Restrepo, M.; Anzueto, A.; et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. Acute Critical Care 2015, 46, 677–686. [CrossRef]
38. Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2021, 384, 693–704, (online ahead of print).
39. Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [CrossRef]
40. Hayakawa, M.; Katabami, K.; Wada, T.; Sugano, M.; Hoshino, H.; Sawamura, A.; Gando, S. Sivelestat (Selective Neutrophil Elastase Inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock 2010, 33, 14–18. [CrossRef]
41. Nakamori, Y.; Park, E.J.; Shimaoka, M. Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front. Immunol. 2021, 11, 624279. [CrossRef] [PubMed]
42. Ryter, S.W. Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders. Antioxidants 2020, 9, 1153. [CrossRef] [PubMed]
43. Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [CrossRef]
44. Cutuli, S.; Grieco, D.; De Pascale, G.; Antonelli, M. Hemadsorption. Curr. Opin. Anaesthesiol. 2021, 34, 113–118, (online ahead of print). [CrossRef] [PubMed]
45. Douvris, A.; Malhi, G.; Hiremath, S.; McIntyre, L.; Silver, S.; Bagshaw, S.M.; Wald, R.; Ronco, C.; Sikora, L.; Weber, C.; et al. Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2018, 22, 41. [CrossRef]
46. Kushimoto, S.; Gando, S.; Saitoh, D.; Mayumi, T.; Ogura, H.; Fujishima, S.; Araki, T.; Ikeda, H.; Kotani, J.; Miki, Y.; et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: An analysis from a multicenter, prospective survey of severe sepsis. Crit. Care 2013, 17, R271. [CrossRef]
47. Malard, B.; Lambert, C.; Kellum, J. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [CrossRef]
48. Broman, M.E.; Hansson, F.; Vincent, J.-L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS ONE 2019, 14, e0220444. [CrossRef]
49. Villa, G.; Romagnoli, S.; De Rosa, S.; Greco, M.; Resta, M.; Montin, D.P.; Prato, F.; Patera, F.; Ferrari, F.; Rotondo, G.; et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: A pilot study. Crit. Care 2020, 24, 1–13. [CrossRef]
50. Cascarano, L.; Cutuli, S.L.; Pintaudi, G.; Tanzarella, E.S.; Carelli, S.; Anzellotti, G.; Grieco, D.L.; DE Pascale, G.; Antonelli, M. Extracorporeal immune modulation in COVID-19 induced immune dysfunction and secondary infections: The role of oXiris® membrane. Minerva Anestesiol. 2021, 87. (online ahead of print). [CrossRef]
51. Harm, S.; Schildböck, C.; Hartmann, J. Cytokine Removal in Extracorporeal Blood Purification: An in vitro Study. Blood Purif. 2020, 49, 33–43. [CrossRef] [PubMed]
52. Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [CrossRef] [PubMed]
53. Seffer, M.-T.; Cottam, D.; Forni, L.G.; Kielstein, J.T. Heparin 2.0: A New Approach to the Infection Crisis. Blood Purif. 2021, 50, 28–34, (online ahead of print). [CrossRef] [PubMed]
54. Livigni, S.; Bertolini, G.; Rossi, C.; Ferrari, F.; Giardino, M.; Pozzato, M.; Remuzzi, G. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: A multicenter randomised controlled clinical trial. BMJ Open 2014, 4, e003536. [CrossRef] [PubMed]
55. Ankawi, G.; Neri, M.; Zhang, J.; Breglia, A.; Ricci, Z.; Ronco, C. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: The promises and the pitfalls. Crit. Care 2018, 22, 262. [CrossRef] [PubMed]
56. Morgera, S.; Slowinski, T.; Melzer, C.; Sobottke, V.; Vargas-Hein, O.; Volk, T.; Zuckermann-Becker, H.; Wegner, B.; Müller, J.M.; Baumann, G.; et al. Renal replacement therapy with high-cutoff hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am. J. Kidney Dis. 2004, 43, 444–453. [CrossRef] Medicina 2021, 57, 552 11 of 11
57. Haase, M.; Bellomo, R.; Baldwin, I.; Haase-Fielitz, A.; Fealy, N.; Davenport, P.; Morgera, S.; Goehl, H.; Storr, M.; Boyce, N.; et al. Hemodialysis Membrane With a High-Molecular-Weight Cutoff and Cytokine Levels in Sepsis Complicated by Acute Renal Failure: A Phase 1 Randomized Trial. Am. J. Kidney Dis. 2007, 50, 296–304. [CrossRef]
58. Morgera, S.; Haase, M.; Kuss, T.; Vargas-Hein, O.; Zuckermann-Becker, H.; Melzer, C.; Krieg, H.; Wegner, B.; Bellomo, R.; Neumayer, H.-H. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit. Care Med. 2006, 34, 2099–2104. [CrossRef]
59. Morgera, S.; Haase, M.; Rocktäschel, J.; Böhler, T.; Vargas-Hein, O.; Melzer, C.; Krausch, D.; Kox, W.J.; Baumann, G.; Beck, W.; et al. Intermittent High-Permeability Hemofiltration Modulates Inflammatory Response in Septic Patients with Multiorgan Failure. Nephron Clin. Pract. 2003, 94, c75–c80. [CrossRef]
60. Morgera, S.; Rocktäschel, J.; Haase, M.; Lehmann, C.; Von Heymann, C.; Ziemer, S.; Priem, F.; Hocher, B.; Göhl, H.; Kox, W.J.; et al. Intermittent high permeability hemofiltration in septic patients with acute renal failure. Intensive Care Med. 2003, 29, 1989–1995. [CrossRef]
61. Antonelli, M.; Bello, G.; Maviglia, R.; Cutuli, S.; Ronco, C.; Cruz, D.; Ranieri, V.M.; Martin, E.; Fumagalli, R.; Monti, G.; et al. Polymyxin B hemoperfusion in clinical practice: The picture from an unbound collaborative registry. Blood Purif. 2014, 37, 22–25.
62. Cutuli, S.L.; Artigas, A.; Fumagalli, R.; Monti, G.; Ranieri, V.M.; Ronco, C.; Antonelli, M. Polymyxin-B hemoperfusion in septic patients: Analysis of a multicenter registry. Ann. Intensiv. Care 2016, 6, 77. [CrossRef]
63. Fujii, T.; Ganeko, R.; Kataoka, Y.; Furukawa, T.; Featherstone, R.; Doi, K.; Vincent, J.; Pasero, D.; Robert, R.; Ronco, C.; et al. Polymyxin B-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018, 44, 167–178. [CrossRef]
64. Chang, T.; Tu, Y.K.; Lee, C.T.; Chao, A.; Huang, C.H.; Wang, M.J.; Yeh, Y.C. Effects of Polymyxin B Hemoperfusion on Mortality in Patients with Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit. Care Med. 2017, 45, e858–e864. [CrossRef] [PubMed]
65. Romaschin, A.D.; Obiezu-Forster, C.V.; Shoji, H.; Klein, D.J. Novel Insights into the Direct Removal of Endotoxin by Polymyxin B Hemoperfusion. Blood Purif. 2017, 44, 193–197. [CrossRef] [PubMed]
66. Srisawat, N.; Tungsanga, S.; Lumlertgul, N.; Komaenthammasophon, C.; Peerapornratana, S.; Thamrongsat, N.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit. Care 2018, 22, 1–10. [CrossRef] [PubMed]
67. De Rosa, S.; Cutuli, S.; Ferrer, R.; Antonelli, M.; Ronco, C.; the COVID-19 EUPHAS2 Collaborative Group. Polymyxin B hemoperfusion in COVID-19 Patients with endotoxic shock: Case Series from EUPHAS II registry. Artif. Organs 2020. (online ahead of print). [CrossRef]
68. Scicluna, B.P.; Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [CrossRef]
69. Celi, L.; Mark, R.; Stone, D.; Montgomery, R. “Big data” in the intensive care unit. Closing the data loop. Am. J. Respir. Crit. Care Med. 2013, 187, 1157–1160. [CrossRef]
70. Mohammed, A.; Van Wyk, F.; Chinthala, L.K.; Khojandi, A.; Davis, R.L.; Coopersmith, C.M.; Kamaleswaran, R. Temporal Differential Expression of Physiomarkers Predicts Sepsis in Critically Ill Adults. Shock 2020. (online ahead of print). [CrossRef]