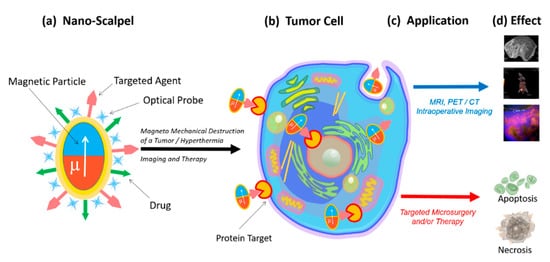
2. Nanoscalpel
Various physical forces can be used to destroy tumor cells, including thermal energy [
4], mechanical energy [
5], and ionizing radiation [
5]. Physical forces trigger mechanisms of tumor cell death via necrosis or apoptosis. The mediator between external forces and the cell should be a structure that transforms one type of energy into another. Magnetic micro- and nanoconstructions are the most suitable structures that convert the energy of a magnetic field into mechanical energy. They can be manipulated in a variety of ways, through motion control, concentration, rotation, oscillation, assembly, or disassembly, using weak magnetic fields [
6].
The magnetic field energy transformation is the most promising option for the destruction of tumor cells, since magnetic fields are safe for humans, and at the same time they can penetrate into the body and control magnetic structures [
7]. Depending on the characteristics of the magnetic field, its energy can be converted into either heat energy, causing the particles heating and hyperthermia of the tumor tissue [
4], or into mechanical energy, causing the oscillation or rotation of the magnetic particles, leading to the mechanically induced cell programmed death or necrotic damage [
8,
9].
In order to perform direct mechanical damage, the torque of the magnetization must be translated into a force applied to the cell, which is capable of damaging the cell membrane [
10,
11] or organelles (in case of penetration of magnetic particles into the cell) [
7,
12,
13,
14,
15].
The magnetic field creates magnetization, which due to magnetic anisotropy, becomes a torque for a physical particle. This torque can be used in a variety of ways. The most attractive way to destroy tumor cells for microsurgery is magnetomechanical destruction due to a magnetic field’s action. Magnetomechanical transduction can act on death receptors [
16], ion channels [
17], or can directly damage the cell [
10,
13,
15]. This effect has been shown both
in vitro [
10,
14] and
in vivo [
7,
12].
That is why recently in biomedical research, magnetic nanoparticles (MNPs) have begun to be actively used. These magnetic nanoparticles are very diverse: (1) they can have different sizes and structures; (2) can be homogeneous or consist of several layers; (3) can have different magnetic properties, depending on the chemical composition, the type of the crystal lattice, and their interactions with neighboring particles [
6].
Superparamagnetic iron oxide nanoparticles (SPION), which exhibit magnetic properties only when a magnetic field is applied, are the most popular ones [
15]. Without a magnetic field, the magnetic moment of the nanoparticles equals zero. Such nanoparticles are often used for magnetic resonance imaging, hyperthermia induction, and mechanical destruction of cells [
4,
15,
18]. However, the effectiveness of these nanoparticles in destroying tumor cells has reached its limit [
19]. Their size restricts the magnitude of the SPION magnetic response required for biomedical applications, since nanoparticles aggregate above the superparamagnetic threshold [
19].
Usually, magnetic nanoparticles have a magnetic core, protective shell, and a biologically functional coating. Ferromagnets, ferrimagnets, and superparamagnets are used as magnetic materials. Various protective materials are used for MNP coatings for biomedical research, since uncoated magnetic particles are unstable under physiological conditions [
20,
21]. In addition, they contribute to the formation of free radicals [
22], can agglomerate [
23], and can be opsonized and captured by macrophages [
24].
For targeted delivery, MNPs are functionalized with ligands capable of specifically binding to target cells. Peptides, antibodies, aptamers, and small molecules with affinity and selectivity are used as carriers. Ligands increase the circulation time of magnetic particles in the blood and improve their biocompatibility [
25,
26,
27].
The use of a nanoparticle-based magnetomechanical approach using a low-frequency, non-heating magnetic field demonstrated the ability of magnetic nanoparticles to convert the energy of a magnetic field into deformation and to create a change in the conformation of macromolecules attached to them [
28]. Thus, MNPs turned out to be capable of manipulating cellular functions with mechanotransduction using remote control of the magnetic field [
29,
30]. In magnetic fields, MNPs can stimulate or suppress cellular functions such as apoptosis, differentiation, migration, proliferation, and secretion [
31,
32]. An alternating magnetic field can cause oscillations of magnetic nanoparticles and can form the basis of magnetomechanical remote destruction of tumor cells [
30]. Successful destruction of tumor cells using MNPs functionalized with antibodies under the action of an alternating magnetic field has been demonstrated
in vitro and
in vivo [
33]. Other studies have demonstrated alternating magnetic fields driving the magnetomechanical
in vivo tumor destruction with aptamer-functionalized MNPs [
34]. However, superparamagnetic nanoparticles are primarily used to induce hyperthermia in tumor tissues [
30,
35,
36,
37,
38]. This approach is based on the high sensitivity of tumor tissues to slight increases in temperature. Heating the tumor up to only +42 °C causes irreversible disruption of the protein conformation due to the tumor tissue’s higher acidity. In contrast, the proteins of normal tissues are insensitive to this temperature [
39]. From a physical point of view, MNPs convert magnetic energy into heat during their magnetization reversal in high-frequency magnetic fields. Magnetic nanoparticles suitable for hyperthermia have a high specific absorption rate (SAR), allowing them to heat up quickly in alternating magnetic fields. However, the efficiency of converting magnetic field energy into thermal energy is low, and the MNPs doses or magnetic anisotropy have to be increased [
40,
41].
An increase in the magnetic moment of the nanoparticle improves the efficiency of the nanoscalpel. However, an increase in the magnetic moment contributes to the undesirable effects of NP aggregation during the preparation of the suspension. In attempting to find a compromised state, it is essential to understand the physical mechanisms involved in the formation of the nanoparticles’ magnetic structure. Additionally, the chemical synthesis of superparamagnetic nanoparticles remains very difficult for mass production due to the relatively low yield and poor reproducibility of the quality of the nanoparticles.
The search for new concepts and strategies has shown that magnetic discs are the most promising magnetic structures for the magnetomechanical destruction of tumor cells. Magnetic discs represent a new generation of particles that can solve biomedical problems in cancer treatment. Magnetic micro- and nanodiscs are characterized by a high saturation of magnetization and the absence of residual magnetization, facilitating remote control of particles with a magnetic field. These properties help to avoid disc agglomeration and make the discs ideal magnetomechanical actuators for disrupting the integrity of cancer cells [
20].
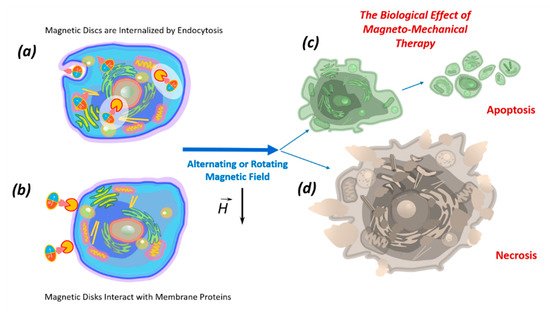
4. Biological effects of discs on tumor cells in an alternating or rotating magnetic field
A fundamental property of all living organisms is mechanosensitivity, which allows cells to perceive physical signals and mechanical forces generated in their microenvironment. This technique controls the parameters of the internal and external environment of the body. Mechanoreceptors control the functional state of cells, tissue growth, stem cell differentiation, apoptosis and necrosis [54,55,56]. Therefore, the mechanosensitivity of cells, including tumor cells, provides the possibility of external control of their functional state.
Vortex and artificial antiferromagnetic (SAF, P-SAF) magnetic nano- and microdisks and disks with a flat quasi-dipole magnetic structure are promising tools for remote control of the functional state of cells. In a low frequency rotating or alternating magnetic field, discs can convert magnetic energy into mechanical energy. Currently, micro- and nanodiscs with zero magnetization have begun to be used in experiments to modulate cell function in the process of bone tissue regeneration [32] and destruction of tumor cells [10].
Data on the ability of vortex and artificial antiferromagnetic discs and discs with a flat quasi-dipole magnetic structure to stimulate the death of tumor cells under the influence of a magnetic field
in vitro and
in vivo are summarized in . Discs have zero total magnetization in the absence of a magnetic field and high magnetization under weak external magnetic fields [
10]. This property ensures the high sensitivity of discs to magnetic stimuli. Various magnetic field parameters are used for magnetomechanical therapy; in particular, the magnetic field strength varies from 5 mT to 1 T, and the frequency of the alternating field is 10–50 Hz. Therapy duration ranges from 1 min to 2 h. At the same time, the biological effect of the applied therapy is practically the same (). Apparently, a short time is sufficient for the destruction of tumor cells using nanodiscs in an alternating magnetic field.
Table 1. The biological effect of magnetic antiferromagnetic (SAF and P-SAF) and vortex Py nano- and microdiscs and discs with a flat quasi-dipole magnetic structure.
| Size |
Disc Type |
Composition |
Magnetic Field Characteristics |
Biological Effect In Vitro |
Reference |
| 2 μm |
P-SAF |
CoFeB connected by Pt/Ru/Pt spacers |
Rotating magnetic field, 10 kOe
Duration 1 min
Torque 18 nN |
Destruction of 62% of U87 cells |
[53] |
| 2 μm |
Py |
Ni80Fe20 |
Rotating magnetic field, 10 kOe
Duration 1 min
Torque 75 nN |
Destruction of 12% U87 cells |
[53] |
| 150/200/350 nm |
Py |
Ni80Fe20 |
Alternating magnetic field, 20 HzDuration 2 h |
Destruction of 83.4/83.2/82.5% of HeLa cells |
[49] |
| 2 μm |
P-SAF |
(Ta/Pt/CoFeB/Pt/Ru/
PT/CoFeB)10 |
Rotating magnetic field, 1 T
Duration 20 min |
Destruction of 70% of U87 cells |
[7] |
| 1.3 μm |
Py |
Ni80Fe20 |
Rotating magnetic field, ∼20–30 mT, 20 Hz
Duration 1 h |
Destruction of 70% of renal cancer cells |
[17] |
1 μm
thickness 60 nm |
Py |
Ni80Fe20 |
Rotating magnetic field, 9 mT 20 Hz
Duration 10 min |
Destruction of 90% of human glioma tumor cell line No. 10 cells (N10) |
[10] |
| 2 μm |
Py |
Ni80Fe20 |
Rotating magnetic field 1 T, 20 Hz,
Duration 30 min |
Destruction of 60% of U87 cells.
In vivo
survival is 3 times higher, and
the tumor is 3 times smaller |
[7] |
| 0.14 μm |
Py |
Ni80Fe20 |
Rotating magnetic field
10 mT, 20 Hz
Duration 30 min |
Destruction of 60% of cells |
[57] |
| 2 μm |
Py |
Ni80Fe20 |
Rotating magnetic field
10 mT, 20 Hz
Duration 30 min |
Destruction of 12% of cells |
[57] |
| 1 μm |
Discs with a flat quasi-dipole magnetic structure |
Au/Ni/Au |
Rotating magnetic field, 50 Hz
5 mT
Duration 20 min
In vitro
In vivo |
Destruction of 80% of Ehrlich ascites adenocarcinoma cell |
[18] |
| 1 μm |
Discs with a flat quasi-dipole magnetic structure |
Au/Ni/Au |
Alternating magnetic field, 50 Hz, 5 mT
Duration 20 min |
Destruction of 90% of Ehrlich ascites adenocarcinoma cell |
[45] |
Kim
et al. showed for the first time that in a rotating magnetic field, vortex discs (20:80 iron-nickel (permalloy) coated with 5 nm Au) destroy 90% of human glioma tumor cell line No. 10
in vitro [
10]. Subsequently, the ability of vortex microdiscs to promote tumor cell death was demonstrated by other authors both
in vitro and
in vivo [
7,
14,
17]. Simultaneously, vortex nanodiscs (140 nm) were more effective in the destruction of tumor cells than vortex microdiscs (1 μm) [
57].
The ability to stimulate tumor cell death under the influence of magnetic fields was found in the vortex and antiferromagnetic discs (SAF and P-SAF). Comparing the efficiency of a vortex and P-SAF discs to destroy tumor cells, Mansell
et al. showed [
53] that P-SAF discs are five times more efficient than vortex discs. The calculations showed that in the case of a rotating magnetic field, microdiscs with uniaxial anisotropy provide continuous torque, in contrast to microdiscs with a plane of easy magnetization, which happens when this effect occurs temporarily when the field is applied.
The study of the dependence of the magnetic discs’ efficiency on the frequency of the applied magnetic field showed that the optimal effect is achieved at frequencies of 10 and 20 Hz (∼90% of cell death). Increasing the frequency to 40 Hz reduced the cell mortality rate to ~75%, while at 50 Hz the cell mortality rate was only ~25%; at 60 Hz, the effect of cell death was absent [
10]. The effect of lower frequencies (from 1 to 20 Hz) was evaluated by Wong
et al. [
49], who showed a slight increase in the lethal effect, with a decrease in frequency from ~80% of viable cells at 10 Hz to ~73% of viable cells at 1 Hz.
7. Impact of Discs on the Cell Membrane
The effects of the discs on the cell membrane of tumor cells and their internalization into the cell occur due to their binding to membrane proteins. This effect of the magnetic discs on tumor cells induces programmed cell death (apoptosis) [
10,
58,
59]. The binding of discs to the cell membrane occurs due to their functionalization via recognition molecules — either antibodies or aptamers. Antibodies to carbonic anhydrase CA9, which is overexpressed on the cell surfaces of solid tumors, were used to functionalize the discs. Magnetic discs with anti-CA9 induced apoptosis in 70% of tumor cells under the influence of a magnetic field [
17]. Using magnetic discs functionalized with antibodies to IL13α2R, other authors changed the homeostasis of calcium cations in tumor cells, stimulating apoptosis [
10]. In this case, the stimulation of mechanosensitive cell receptors using vibrating discs caused the cell membrane to stretch, which led to an increase in the intracellular level of calcium cations [
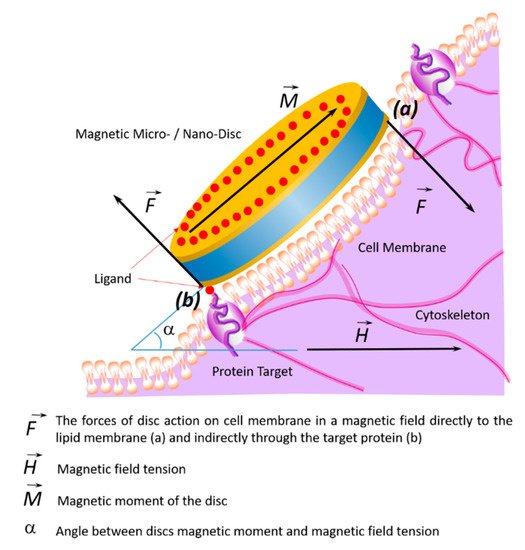
59]. Presumably, the cytoskeleton, which responds to external or internal physical stimuli and is responsible for cellular mechanical signal transmission, cell shape regulation, and migration, plays a key role in controlling the cell’s functional state in external magnetic fields. A schematic diagram of the target cell’s functional state is shown in , showing the action of magnetic discs on membrane proteins remotely controlled by an alternating or rotating magnetic field.
Figure 4. Schematic diagram showing changes of the target cell’s functional state under the influence of magnetic discs and the effects on membrane proteins in an alternating or rotating magnetic field. In a magnetic field, the discs’ forces act on the cell membrane and cytoskeleton in two ways: directly (a) or indirectly through the target protein (b).
9. Transfer of Magnetic Discs along with the Bloodstream
In the bloodstream, anisotropic magnetic discs tend to deflect towards the vessel wall and carry out lateral drift along streamlines to the endothelium due to inertial and hydrodynamic forces [
61]. This leads to the adhesion of particles to the endothelium near the tumor site, facilitating their diffusion from the blood vessels into the tumor tissue according to the “effect of increased permeability and retention (EPR)” in the tumor.
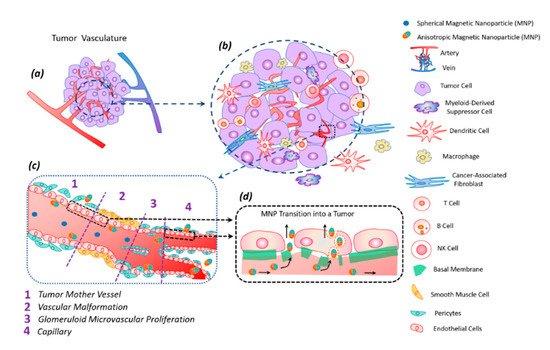
The tumor tissue has an altered vasculature [
60] (). Tumor mother vessels are characterized by thinning or contraction of endothelial cells, basal membrane degradation, and pericytes detachment (c1). As a result of these structural changes, tumor vessels become highly permeable for small and large molecules and particles. These vessels are unstable and eventually differentiate into glomeruloid microvascular proliferation (c2), vascular malformations (c3), and capillaries (c4). Glomeruloid microvascular proliferations are tortuous vessels composed of irregular layers of pericytes and endothelial cells with multiple very small vascular lumens (c2). Vascular malformations are similar in size to the mother vessel but with a smooth muscle covering (c3). Capillaries are formed from mother vessels and glomeruloid microvascular proliferation (c4). Mother vessels can also include holes and open pores through which macromolecules can extravasate. In the extravasation mechanism of interendothelial cells, nanoparticles are transported through gaps measuring 100–500 nm in diameter [
60].
Figure 5. Tumor vasculature (a) in a complex tumor microenvironment (transformed and immune cells) (b). Transport of magnetic anisotropic discs and spherical magnetic nanoparticles along transformed tumor vessels (c). Spherical particles move in the central part of the vascular bed, while anisotropic magnetic discs move along the periphery of the vessel, frequently interacting with the vessel wall; this peculiarity facilitates discs transmigration into the tumor (c). Magnetic nanodiscs are able to pass into a tumor through the damaged basal membrane in two ways: through endothelial cells and the gaps between them (d).
The anisotropic shape of the magnetic particle facilitates its better penetration into the tumor [
62]. The vibration of anisotropic particles due to hydrodynamic or magnetic forces causes enhanced particle interactions with the vessel wall and transmigration into the tumor [
43,
62]. The vascular density of a tumor is usually the highest at the tumor–host interface. Central parts of tumors tend to be less vascularized, which often have zones of necrosis due to insufficient blood supply. Plasma proteins increase the viscosity of the blood, slowing down the blood flow. For this reason, nanoparticles within the tumor vessels tend to move slowly or stagnate. Therefore, nanoparticles have enough time to diffuse from the vessel into the tumor’s extracellular matrix. Size is an important parameter for magnetic particles—they should be small enough not to clog blood microcapillaries and pass through the pores of blood vessels in order to diffuse into tissues.
10. Modification and Functionalization of Discs
The stability of magnetic particles in suspension depends on hydrophobic–hydrophilic and van der Waals forces. Magnetic discs have a large surface area to volume ratio, capable of forming micrometer-sized clusters [
43]. To reduce cluster formation, surface modification with surfactants, natural dispersants, organic dyes, or polymers is required. This makes magnetic particles more stable in biological media. The dispersibility of magnetic particles can be improved using silica and gold. However, the use of non-magnetic materials to coat magnetic particles can lead to a decrease in magnetization saturation. To optimize the characteristics of disc-shaped magnetic particles, a compromise should be found between surface modification and retention of magnetic properties [
43].
The stability of magnetic discs could also be increased through their functionalization with specific ligands, promoting targeted action on a tumor cell. Additionally, to reduce side effects, the magnetic particles must only enter the tumor cells. Additional functionalization with the ligands specific only to tumor cells, such as vascular endothelial growth factor receptor (VEGFR), transferrin receptors, or integrins, is required for the targeted action [
63,
64]. Additionally, the optimization of the target biodistribution of magnetic discs in vivo will be determined by the local blood flow, pH, organization of the vascular network, and extracellular matrix.
Consequently, specific targeting is necessary to increase the efficiency of the delivery of magnetic discs to the tumor. This can be achieved by using ligands that are complementary to target sites, which can be peritumoral and intratumoral blood vessels, the extracellular matrix, tumor cells, or intracellular targets with non-specific (passive) aim. To perform functionalization, the surfaces of nanoparticles are covered only with stabilizing agents. The functionalization of magnetic particles with ligands specific for tumor cells will increase their accumulation in the tumor tissues. The biological effects of magnetic discs functionalized with tumor cell-specific ligands (antibodies or aptamers) are shown in .
Table 2. The biological effects of magnetic discs functionalized with antibodies and aptamers.
| Disc Type |
Recognizing Agent |
Disc Binding to Recognizing Agent |
Cell Type; Destruction Rate |
Reference |
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs, coated with a 5-nm-thick layer of gold on each side |
Antibodies
anti-IL13α2R |
S–Au bond |
Human glioma N10 cell line; 90% (in vitro) |
[10] |
| The 60-nm-thick, ~1-μm-diameter 20:80% iron–nickel (permalloy) discs |
Antibody antihCA9 |
S–Au bond |
Renal SCRC-59 renal cancer line; 90% (in vitro) |
[17] |
Discs with a flat quasi-dipole magnetic structure
Au/Ni/Au |
Aptamer |
S–Au bond |
Ehrlich ascites adenocarcinoma cell line; 80% (in vitro, in vivo) |
[18] |
Discs with a flat quasi-dipole magnetic structure
Au/Ni/Au |
Aptamer |
S–Au bond |
Ehrlich ascites adenocarcinoma cell line;
90% (in vitro, in vivo) |
[45] |
Aptamers are one of the preferable molecules as ligands for targeting due to the following properties: (1) aptamers can be selective to any desired target; (2) similarly to antibodies, aptamers have high specificity and high affinity to their target; (3) aptamers are manufactured chemically in an easily scalable process; (4) the chemical process for the production of aptamers is not susceptible to viral or bacterial contamination; (5) aptamers are non-immunogenic and non-toxic; (6) the small size of the aptamers allows them to penetrate effectively into any tumors; (7) aptamers can reversibly denature with the restoration of the desired conformation, while the phosphodiester bond is chemically stable; (8) aptamers can be chemically modified without changing their conformation; (9) chemical compounds for the addition of dyes or functional groups can be easily introduced during synthesis [
65].