Inositol 1,4,5-triphosphate receptor-associated cGMP kinase substrate 1 (IRAG1) is a substrate protein of the NO/cGMP-signaling pathway and forms a ternary complex with the cGMP-dependent protein kinase Iβ (PKGIβ) and the inositol triphosphate receptor I (IP3R-I).
- anemia
- IP3R-I
- iron deficiency
- IRAG
- IRAG1
- MRVI1
- PKGI
- PKGIβ
1. Introduction
The inositol 1,4,5-triphosphate receptor-associated cGMP kinase substrate 1 (IRAG1), which is also named IRAG or as human homologue MRVI1, was first described in 2000 [1].
IRAG1 is a substrate protein of the NO/cGMP-signaling pathway. Molecular analysis showed that IRAG1 has a molecular weight of 125 kDa and forms a ternary complex with the cGMP-dependent protein kinase Iβ (PKGIβ) and the inositol triphosphate receptor I (IP3R-I) [1]. An investigation of the stability of this complex confirmed that IRAG1 is of great importance for the integrity of this complex [2][3]. IRAG1 is only phosphorylated by PKGIβ and not by PKGIα or PKGII and interacts with the IP3R-I [2]. This phosphorylation of IRAG1 and the interaction with the IP3R-I lead to a reduction of the cGMP-mediated IP3-dependent Ca2+ release, which was shown in cellular systems and in the Irag1Δ12/Δ12 mice [2][3][4]. In these mice, exon 12 of IRAG1 is deleted, which is coding for the N-terminus of the coiled-coil domain and resulted in a disrupted in vivo interaction between IRAG1 and IP3R-I.
Protein expression of IRAG1 is widespread in murine tissue. Smooth muscle containing tissues and platelets highly expressed IRAG1, while lower amounts of this protein were found for example in the spleen. PKGIβ correlated with IRAG1 in the amount of expression and tissue distribution [5].
Most of the pathophysiological functions of IRAG1 are still unknown. Some physiological functions are the inhibition of NO/cGMP-dependent platelet aggregation [6][7], thrombus formation [6] and regulation of the smooth muscle relaxation [4][8]. In these previous investigations some pathophysiological effects were found. Irag1Δ12/Δ12 mice developed an enlarged gastrointestinal tract and showed a reduced passage time, which demonstrated a stenosis of the pylorus and other functional disorders of gastrointestinal motility [4]. This gastrointestinal phenotype was also found in global Irag1−/− mice [8] and in both mutant mice (Irag1Δ12/Δ12 and Irag1−/−) indications of an enlarged spleen were found. In summary, a defect or loss of IRAG1 causes gastrointestinal disorders.
The central part of the NO/cGMP-signaling pathway is related to PKGI. It was reported that a loss of the PKGI in global PKGI-KO mice (genotype: Prkg1−/−) showed gastrointestinal disorders as enlargement of the gastrointestinal tract and reduced passage time [9]. Further research about the pathophysiological function of the PKGI revealed that Prkg1−/− mice developed anemia and splenomegaly [10] and gastrointestinal bleedings caused by duodenal ulcers [11]. Treatment with a proton pump inhibitor (PPI) reduced the gastrointestinal bleeding [11][12] and the development of splenomegaly [12].
2. Results
2.1. Detection of Occult Blood in IRAG1-WT and IRAG1-KO Mice
The proof of blood in feces is an indication of gastrointestinal bleeding. A fast and secure method for the diagnostic of (occult) blood in the feces is the Haemoccult® Test, a frequently used tool in human diagnostics. As there might be the possibility that Irag1-deficient mice may develop gastrointestinal bleeding caused by the similarities of the phenotype to the global Prkg1-KO mice [4][8][9], the feces of 14-week-old male and female IRAG1 knockout (KO) mice (IRAG1-KO, genotype: Irag1−/−) and their wild type (WT)-littermates (IRAG1-WT, genotype: Irag1+/+) were investigated (Figure 1). In total, 81% of female IRAG1-KO mice had a positive result, whereas no Haemoccult® Tests of female IRAG1-WT mice were positive. In the group of the male mice, 31% of IRAG1-KO mice had occult blood in the feces, and 8% of IRAG1-WT mice showed a positive result. The total result of both sexes presented similar results; in 56% of IRAG1-KO mice and in 5% of IRAG1-WT mice, we detected blood in the feces. To figure out if these findings are age-dependent, we analyzed the feces of six-week-old IRAG1-KO mice and their WT littermates too, and no blood was detected in the feces.
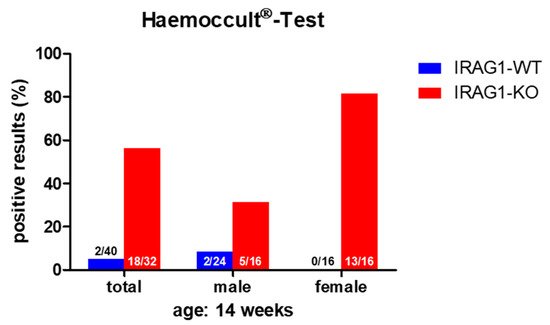
Figure 1. The Haemoccult® Test of IRAG1-WT and IRAG1-KO mice. Occult blood was found in the IRAG1-KO mice, but with a difference in positive results between male and female IRAG1-KO mice. The numbers in the graphs indicated the mice that had a positive Haemoccult® Test compared to the total number of investigated mice; “total” indicates the evaluation of both sexes (male and female) from each genotype.
Therefore, IRAG1-KO mice contained occult blood in the feces, which may be caused by gastrointestinal bleeding.
2.2. IRAG1-Deficient Mice Develop Splenomegaly
IRAG1 and PKGIβ expression largely overlap at the protein level [5], and IRAG1 mutant mice have a similar phenotype, e.g., the dilatation of the gastrointestinal tract [4][8], reduced gastrointestinal motility [4] and bleeding (Section 2.1, Figure 1) similar to the global Prkg1-KO mice [9], and it is known that Prkg1-deficient mice develop splenomegaly [10][12]. To investigate if Irag1-deficiency caused an enlarged spleen, the weights of the spleen and body were determined, and the ratio between these weights (spleen/body-ratio) was calculated.
A lack of IRAG1 caused a significant increase in spleen weights of the female IRAG1-KO mice compared to their WT littermates (Table 1, Figure 2A), and the same result was found in the total analysis of both sexes. Between male IRAG1-WT and their KO-littermates, no differences were found. The explored body weight of the genotypes and sexes differed insignificantly (Table 1, Figure 2B) and thus, did not influence the calculation of the spleen-weight-to-body-weight ratio. The spleen/body-ratio calculated from these weights was significantly increased in the female IRAG1-KO mice and in the total analysis of both sexes compared to the corresponding IRAG1-WT (Table 1, Figure 2C). No significant difference in this ratio was found between male IRAG1-WT and IRAG1-KO mice. In 7-week-old Irag1-deficient mice, no differences were detected in these parameters compared to their WT littermates.
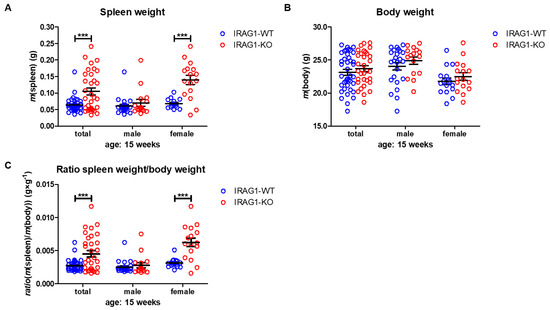
Figure 2. Analysis of spleen and body weight of IRAG1-WT and IRAG1-KO mice. (A) Increased spleen weights in IRAG1-KO (total: n = 32; female: n = 16) mice compared to their WT littermates (total: n = 40; female: n = 16) were determined, but not in male IRAG1-KO (n = 16) and IRAG1-WT mice (n = 24). (B) No differences in the body weights of IRAG1-KO mice (total: n = 32; male: n = 16; female: n = 16) and their WT littermates (total: n = 40; male: n = 24; female: n = 16) were found. (C) Spleen-weight-to-body-weight ratios of IRAG1-KO mice (total: n = 32; female: n = 16) were increased compared to their WT littermates (total: n = 40; female: n = 16), but not in male IRAG1-KO (n = 16) and IRAG1-WT mice (n = 24). Circles indicate the individual value of each mouse and mean ± SEM is shown by bars. Significant differences are shown by (***) (p < 0.001).
| Parameter | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | |
| spleen weight (g) | 0.0634 ± 0.0034 | 0.1051 ± 0.0106 a | 0.0605 ± 0.0050 | 0.0702 ± 0.0103 | 0.0678 ± 0.0035 | 0.1399 ± 0.0140 a |
| body weight (g) | 23.13 ± 0.43 | 23.70 ± 0.45 | 24.04 ± 0.57 | 24.91 ± 0.55 | 21.77 ± 0.51 | 22.49 ± 0.58 |
| spleen/body-ratio (g × g−1) | 0.0028 ± 0.0001 | 0.0045 ± 0.0005 a | 0.0025 ± 0.0002 | 0.0028 ± 0.0004 | 0.0031 ± 0.0002 | 0.0062 ± 0.0007 a |
These results demonstrate that Irag1-deficiency led to an increase in the spleen weight and spleen-to-body-weight ratio. This phenotype was mainly observed in female IRAG1-KO mice.
2.3. Analysis of Hematological Parameters
To assess whether the gastrointestinal bleeding resulted in hematological abnormalities, we investigated in the following step the hematological status. Therefore, we characterized some basic hematological parameters, with the aim to obtain an overview of the hematological status of Irag1-deficient mice.
Hematocrit (HCT) values were significantly reduced in female IRAG1-KO mice and in the total analysis of both sexes, while in male IRAG1-KO mice, no reduction was found (Table 2, Figure 3A). The number of erythrocytes/red blood cells (RBCs) was likewise significantly decreased in the total analysis and in female Irag1-deficient mice. Again, no significant difference was found in male IRAG1-KO compared to their WT littermates (Table 2, Figure 3B). A different result was found regarding the hemoglobin (Hb). In both sexes and in the total analysis of Irag1-deficient mice, significantly lower Hb levels were detected in contrast to IRAG1-WT mice (Table 2, Figure 3C). The mean corpuscular volume (MCV) (Table 2, Figure 3D) and mean corpuscular hemoglobin (MCH) (Table 2, Figure 3E) were elevated in female IRAG1-KO mice significantly and MCV in the total analysis in IRAG1-KO as well. Conversely, no differences in MCV and MCH were found in male mice between both genotypes. Finally, we analyzed the numbers of leukocytes/number of white blood cells (WBC), and we found no differences between both genotypes in both sexes (Table 2, Figure 3F). Seven-week-old IRAG1-WT and IRAG1-KO mice have no differences in these parameters. Old IRAG1-KO mice (age: 54–62 weeks) developed anemia and had an increase in the number of reticulocytes and the reticulocytes production index (RPI).
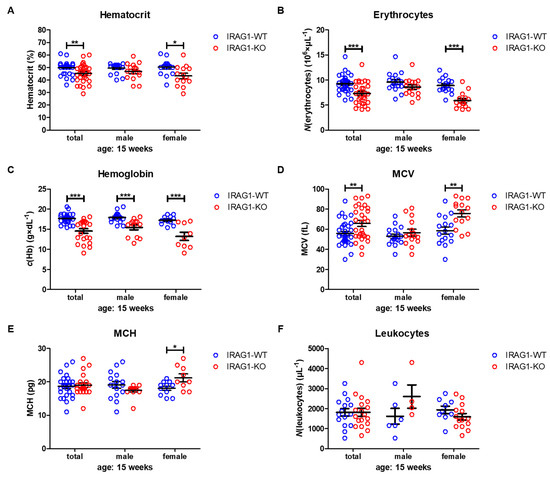
Figure 3. Hematological parameters of IRAG1-WT and IRAG1-KO mice. (A) Significant reduction of hematocrit in IRAG1-KO mice (total: n = 29; female: n = 14) in comparison to IRAG1-WT mice (total: n = 33 and female: n = 16) and no differences were detected in male IRAG1-WT (n = 17) and IRAG1-KO mice (n = 15). (B) Irag1-deficiency reduced RBCs in IRAG1-KO (total: n = 29; female: n = 14) and had no effect in male IRAG1-KO mice (n = 15) as compared with their WT littermates (total: n =33; male: n = 17; female: n = 16). (C) Significantly affected hemoglobin values of IRAG1-KO mice (total: n = 22; male: n = 13; female: n = 9) by the Irag1-deficiency compared to WT littermates (total: n = 27; male: n = 16; female: n = 11). (D) MCV was significantly increased in IRAG1-KO mice (female: n = 14; total: n = 29) compared to IRAG1-WT (female: n = 16; total: n = 33), and no difference was found between male IRAG1-WT (n = 17) and IRAG1-KO mice (n = 15). (E) MCH was not affected by Irag1-deficiency in male mice (IRAG1-WT: n = 16; IRAG1-KO: n = 13) and in total (IRAG1-WT: n = 27; IRAG1-KO: n = 22). Female IRAG1-KO mice (n = 9) developed a significantly higher MCH compared to their WT littermates (n = 11). (F) WBCs were not different between IRAG1-WT mice (total: n = 15; male: n = 6; female: n = 9) and IRAG1-KO mice (total: n = 18; male: n = 4; female: n = 14). Circles indicate the individual value of each mouse and mean ± SEM is shown by bars. Significant differences are shown by (*) (p < 0.05), (**) (p < 0.01) and (***) (p < 0.001).
Table 2. Hematological parameters of IRAG1-WT and IRAG1-KO mice.
| Parameter | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | |
| HCT (%) | 49.9 ± 0.9 | 45.1 ± 1.4 b | 49.5 ± 1.0 | 46.9 ± 1.7 | 50.3 ± 1.5 | 43.3 ± 2.2 a |
| RBC (106 × µL−1) | 9.27 ± 0.30 | 7.28 ± 0.41 c | 9.60 ± 0.46 | 8.58 ± 0.47 | 8.92 ± 0.38 | 5.90 ± 0.43 c |
| Hb (g × dL−1) | 17.6 ± 0.2 | 14.5 ± 0.6 c | 17.9 ± 0.3 | 15.5 ± 0.6c | 17.2 ± 0.3 | 13.2 ± 1.0 c |
| MCH (fL) | 55.7 ± 2.1 | 65.8 ± 3.0 | 53.0 ± 2.2 | 56.6 ± 3.4 | 58.6 ± 3.7 | 75.7 ± 3.6 a |
| MCV (pg) | 18.8 ± 0.6 | 19.1 ± 0.8 b | 19.2 ± 4.0 | 17.5 ± 0.5 | 18.2 ± 0.6 | 21.3 ± 1.1 b |
| WBC (µL−1) | 1807 ± 189 | 1811 ± 199 | 1617 ± 393 | 2600 ± 582 | 1933 ± 188 | 1586 ± 162 |
Taken together, the Irag1-deficiency caused severe anemia, as shown in the reduction of all important blood parameters. In this context, it was interesting to see that anemia was more prominent in female IRAG1-KO mice than in male IRAG1-KO mice.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22115458
References
- Schlossmann, J.; Ammendola, A.; Ashman, K.; Zong, X.; Huber, A.; Neubauer, G.; Wang, G.X.; Allescher, H.D.; Korth, M.; Wilm, M.; et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 2000, 404, 197–201.
- Ammendola, A.; Geiselhöringer, A.; Hofmann, F.; Schlossmann, J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Iβ. J. Biol. Chem. 2001, 276, 24153–24159.
- Fritsch, R.M.; Saur, D.; Kurjak, M.; Oesterle, D.; Schlossmann, J.; Geiselhöringer, A.; Hofmann, F.; Allescher, H.-D. InsP3R-associated cGMP kinase substrate (IRAG) is essential for nitric oxide-induced inhibition of calcium signaling in human colonic smooth muscle. J. Biol. Chem. 2004, 279, 12551–12559.
- Geiselhöringer, A.; Werner, M.; Sigl, K.; Smital, P.; Wörner, R.; Acheo, L.; Stieber, J.; Weinmeister, P.; Feil, R.; Feil, S.; et al. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 2004, 23, 4222–4231.
- Geiselhöringer, A.; Gaisa, M.; Hofmann, F.; Schlossmann, J. Distribution of IRAG and cGKI-isoforms in murine tissues. FEBS Lett. 2004, 575, 19–22.
- Antl, M.; von Brühl, M.-L.; Eiglsperger, C.; Werner, M.; Konrad, I.; Kocher, T.; Wilm, M.; Hofmann, F.; Massberg, S.; Schlossmann, J. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood 2007, 109, 552–559.
- Schinner, E.; Salb, K.; Schlossmann, J. Signaling via IRAG is essential for NO/cGMP-dependent inhibition of platelet activation. Platelets 2011, 22, 217–227.
- Desch, M.; Sigl, K.; Hieke, B.; Salb, K.; Kees, F.; Bernhard, D.; Jochim, A.; Spießberger, B.; Höcherl, K.; Feil, R.; et al. IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc. Res. 2010, 86, 496–505.
- Pfeifer, A.; Klatt, P.; Massberg, S.; Ny, L.; Sausbier, M.; Hirneiß, C.; Wang, G.X.; Korth, M.; Aszódi, A.; Andersson, K.E.; et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998, 17, 3045–3051.
- Föller, M.; Feil, S.; Ghoreschi, K.; Koka, S.; Gerling, A.; Thunemann, M.; Hofmann, F.; Schuler, B.; Vogel, J.; Pichler, B.; et al. Anemia and splenomegaly in cGKI-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 6771–6776.
- Singh, A.K.; Spießberger, B.; Zheng, W.; Xiao, F.; Lukowski, R.; Wegener, J.W.; Weinmeister, P.; Saur, D.; Klein, S.; Schemann, M.; et al. Neuronal cGMP kinase I is essential for stimulation of duodenal bicarbonate secretion by luminal acid. FASEB J. 2012, 26, 1745–1754.
- Angermeier, E.; Domes, K.; Lukowski, R.; Schlossmann, J.; Rathkolb, B.; Angelis, M.H.; Hofmann, F. Iron deficiency anemia in cyclic GMP kinase knockout mice. Haematologica 2016, 101, e48–e51.
