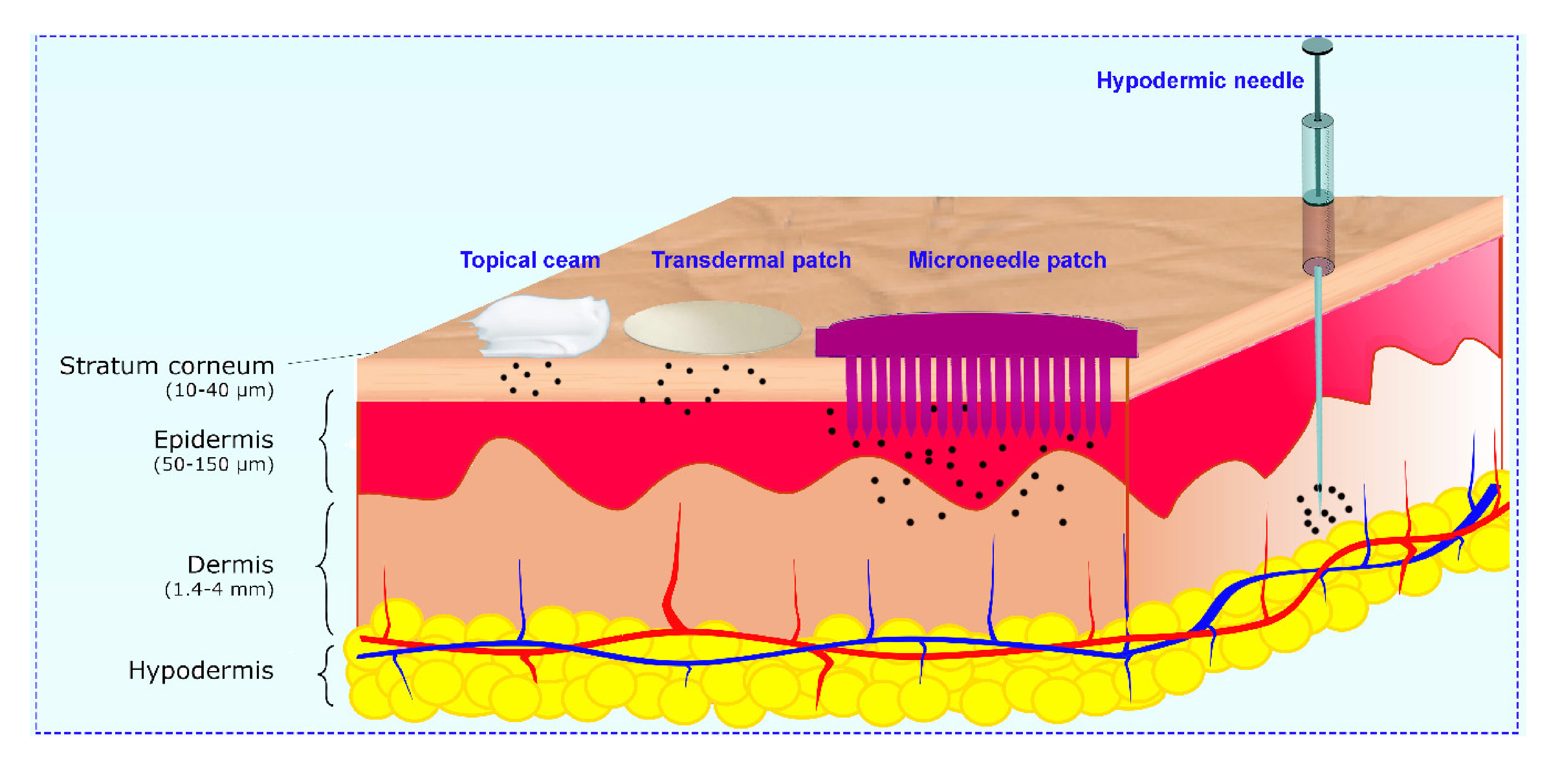
The growing demand for patient-compliance therapies in recent years has led to the development of transdermal drug delivery, which possesses several advantages compared with conventional methods. Delivering protein through the skin by transdermal patches is extremely difficult due to the presence of the stratum corneum which restricts the application to lipophilic drugs with relatively low molecular weight. To overcome these limitations, microneedle (MN) patches, consisting of micro/miniature-sized needles, are a promising tool to perforate the stratum corneum and to release drugs and proteins into the dermis following a non-invasive route.
- Microneedle, Protein delivery, Vaccine delivery
MNs are able to encapsulate proteins with high efficiency and store them in a bioactive state without requesting further expedients (cold-chain), hence minimizing the cost of transportation. Moreover, the addition of excipients, such as ethylene-diaminetetraacetic acid trehalose or mannitol, into their formulation can also stabilize the activity of the drugs over several days. Unfortunately, the current MN preparations still show some limitations. For example, solid MNs have expensive fabrication costs and their difficult application processes are troublesome for patients. Coated MNs are able to load only a small amount of drugs since they can only be applied on the surface of the MNs, while hollow MNs are characterized by a potential toxic effect
because of the uncontrolled drug dose release that occurs, and they require specialized personnel and a complex set up for the injection. In addition, there are dissolving MNs, which are not able to provide prolonged release, and most of the methods are not suitable for protein stability. Despite these restrictions, the progress in MN fabrication and protein encapsulation make microneedles a promising platform for the dermal delivery of drugs.
Type of MNs Based on Their Structure and Release Profile
Hollow MNs
Hollow MNs emerged to inject liquids and suspensions for drug infusion into the body through the needle bore [1]. Silicon, metal, or glass can be employed for the preparation of hollow MNs with an adjustable bore diameter.
Coated MNs
In this kind of patch, drugs are adhered directly onto the surface of solid or polymeric MNs . Since the coating layer reduces the mechanical strength and sharpness of the needles, the drug loading on the needle surface is limited to a low amount. As a consequence, coated MNs are only usable for some specific applications in which a low dose is needed [2].
Dissolvable Matrix MNs
Dissolving MNs were created to encapsulate drugs within a water-soluble polymer matrix, and they become completely dissolved once the MNs are inserted into the skin, with a dissolving time from minutes to hours [3].
Degradable Particle Embedded-MNs
In order to achieve sustained drug release, MNs with biodegradable polymer particles were designed. The drug payload is gradually released by simple diffusion and hydrolysis of the polymer [4].
Swellable MNs
Swellable MNs, also known as cross-linked hydrogels, are solid systems that swell after the uptake of interstitial fluid (IF), after which they deliver the payload, and are removed intact from the skin [5].
Bio-responsive MNs
Bio-responsive materials have the outstanding characteristic of sensing physiological or pathological signals and regulating on-demand release based on the presence and intensity of a specific stimulus [6]. For example, glucose-responsive MNs can sense the concentration of glucose and as a feedback release a proportional amount of insulin. They also can recognize abnormal signals such as low pH in the tumor site and provide targeted cancer therapy. All in all, these smart MNs reduce the risk of side effects and prevent drug waste while increasing the efficacy of the therapy by liberating a specific amount of drug exactly where and when it is needed.
Conclusion
The clinical development of proteins is faster than that of small-molecules or peptides, although their chemical-physical properties put wide restrictions on transdermal delivery. Particularly, proteins are subject to degradation in aqueous environments, undergoing aggregation, denaturation, or precipitation mechanisms. Microneedles possess many advantages to avoid protein denaturation and aggregation (e.g., rapid delivery) as compared with other systemic administrations, and for this reason, they could significantly revolutionize the field of drug delivery.
References
- Lin Niu; Leonard Y. Chu; Scott A. Burton; Kris J. Hansen; Jayanth Panyam; Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. Journal of Controlled Release 2019, 294, 268-278, 10.1016/j.jconrel.2018.12.026.
- Peter C. DeMuth; Adrienne V. Li; Peter Abbink; Jinyan Liu; Hualin Li; Kelly A. Stanley; Kaitlin M. Smith; Christy L. LaVine; Michael S. Seaman; Joshua A. Kramer; et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nature Biotechnology 2013, 31, 1082-1085, 10.1038/nbt.2759.
- Jeong Woo Lee; Seong-O Choi; Eric I. Felner; Mark R. Prausnitz; Dissolving microneedle patch for transdermal delivery of human growth hormone.. Small 2011, 7, 531-9, 10.1002/smll.201001091.
- Mario Battisti; Raffaele Vecchione; Costantino Casale; Fabrizio A. Pennacchio; Vincenzo Lettera; Rezvan Jamaledin; Martina Profeta; Concetta Di Natale; Giorgia Imparato; Francesco Urciuolo; et al. Non-invasive Production of Multi-Compartmental Biodegradable Polymer Microneedles for Controlled Intradermal Drug Release of Labile Molecules. Frontiers in Bioengineering and Biotechnology 2019, 7, 296, 10.3389/fbioe.2019.00296.
- Eman M. Migdadi; Aaron J. Courtenay; Ismaiel Tekko; Maelíosa T.C. McCrudden; Mary-Carmel Kearney; Emma McAlister; Helen O. McCarthy; Ryan F. Donnelly; Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. Journal of Controlled Release 2018, 285, 142-151, 10.1016/j.jconrel.2018.07.009.
- Guohua Jiang; Bin Xu; Jiangying Zhu; Yang Zhang; Tianqi Liu; Gao Song; Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomedical Physics & Engineering Express 2019, 5, 045038, 10.1088/2057-1976/ab3202.