Surviving negative effects of a wide variety of environmental fluctuations is a substantial part of plant life. Dynamic metabolic, genetic and morphologic changes of plants are necessary to acclimatize and/or adapt to harmful environmental conditions. These changes are controlled by the rapid transient or chronic production and the scavenging of reactive oxygen species (ROS) [
1,
2,
3]. In these processes, several subcellular components have a distinguished role, such as mitochondria [
4,
5,
6]. At the same time, the role of mitochondria can be investigated only together with other organelles (e.g., chloroplasts, nuclei, endoplasmic reticulum) in these processes, which can be mediated by various hormones, such as salicylic acid (SA) [
7,
8,
9].
2. SA and Its Effects on the Structure of Plant Mitochondria and ETC Compartments
ROS metabolism in the mitochondria and redox-mediated signaling cross-talk with plant hormones such as salicylic acid (SA). SA has been described to play an essential role in the regulation of plant defence signaling upon various abiotic and biotic stressors [
78,
79]. It is required for the establishment of both local and systemic acquired resistance (SAR) after a pathogen attack [
80,
81,
82]. It is well known that there is a close correlation between ROS production and changes in the SA content [
83]. Increase in the endogenous concentration of SA under various stress conditions induces the rapid accumulation of ROS, leading to oxidized proteins and cell death in the infected tissues [
78]. However, SA can also induce stress tolerance that is highly dependent on the accumulation of superoxide radicals and H
2O
2, which are essential mediators of hypersensitive reaction (HR) and PCD induction in high concentration. Furthermore, SA-generated ROS could contribute to cellular redox homeostasis through the regulation of the expression and activity of antioxidant enzymes in lower levels [
84]. At the same time, the source of ROS induced by SA could be originated from various cell compartments (e.g., chloroplasts, mitochondria, plasma membrane-localized NADPH oxidase, polyamine oxidase) [
85,
86,
87]. Thus, the effects of different cell organelles to each other can further complicate unravelling the effect of SA. In addition, there are contrasting findings from different experiments in the case of SA-generated ROS. It has to be mentioned, that the action of SA is highly dependent, e.g., on its applied or internal concentrations, on the duration and the mode of the application, on the investigated plant species and organs as well as on environmental (e.g., light intensity) conditions [
79,
80]. Furthermore, the crosstalk between SA and other plant hormones (e.g., jasmonic acid and ethylene) can modify the defence reactions and PCD by regulating ROS metabolism [
88]. In this section, the SA-generated mitochondrial ROS production, scavenging and signaling of mitochondrial ROS are summarized to understand the role of this important phytohormone in these processes.
SA can affect plant mitochondrial morphology and function in a dose- and time-dependent manner [
7,
9,
87,
89,
90]. First of all, a rapid and significant change in mitochondrial morphology was observed in response to 0.5 mM SA in
Arabidopsis protoplasts [
8]. Authors found that tens of mitochondria arranged into clusters and the individual mitochondria became swollen within 40 min upon SA. After 1 h, a more irregular clumped or clustered morphology of mitochondria was observed in SA-treated protoplasts. Interestingly, the application of AsA before SA exposure reduced these alterations in mitochondrial morphology, which demonstrated that the aggregation of mitochondria is highly dependent on the production of mitochondrial ROS [
8]. In intact tomato mesophyll cells, exogenous SA treatments caused swelling and disorganization of mitochondrial cristae as well as disintegration and vacuolization of mitochondria, which proved to be more serious at lethal (1 mM) SA concentration after 24 h [
91]. Changes in mitochondrial morphology are related to physiological function and energy metabolism [
92], which are important steps of plant PCD [
43,
44,
45,
46]. High SA concentration can cause the loss of mitochondrial integrity and cyt
c release from mitochondria as well as ROS production and membrane lipid peroxidation, which take place before PCD execution [
5,
92]. At the same time, the long-term effect of sublethal concentration of SA on mitochondria biogenesis, number, structure and contact with other organelles have not been analyzed yet. Among others, investigation of prohibitins, which play role in the biogenesis and protection of mitochondria could be an interesting research field. Namely, earlier it was found that the suppression of prohibitin function resulted in a 10- to 20-fold higher ROS production and premature leaf senescence in
Nicotiana benthamiana, and these plants were more susceptible to SA [
93].
It has been also confirmed that SA by concentration- and time-dependent manner impact on mitochondria ETC by inhibiting the mitochondrial ETC and oxidative phosphorylation [
7]. Firstly, it was found that 0.02–0.5 mM SA induced inhibition of both respiratory O
2 uptake and ATP synthesis within minutes after SA incubation in tobacco cell suspension cultures. This effect of SA was reduced by the application of the antioxidant N-acetylcysteine, suggesting a possible role for ROS in the SA-mediated inhibition of mitochondrial functions [
89]. Later, inhibition of O
2 uptake of purified soybean cell mitochondria was also observed after 16 h-long-treatment with 1 mM SA [
94]. Norman et al. [
7] described firstly that SA at low concentrations (0.1–0.5 mM) acted as an uncoupler, whereas SA at higher concentrations (1–5 mM) strongly inhibited mitochondrial electron flow in tobacco cell suspension culture. Initially, they measured that SA blocks electron flow from the substrate dehydrogenases to the UQ pool [
7]. Shugaev et al. [
90] observed that by using stored taproots of sugar beet (
Beta vulgaris L.) and etiolated seedling cotyledons of yellow lupine (
Lupinus luteus L.), the uncoupling action on mitochondrial respiration and dissipation of mitochondrial membrane potential upon SA treatment was not only dependent on SA concentration but also on the duration of the treatment and on the sensitivity of mitochondria isolated from different plant tissues to the phytohormone. However, the direct effect of SA on ETC components remained unknown. Complex I and III of ETC are considered to the major sites of ROS production. Namely, over-reduction of ETC components and accumulation of mitochondrial ROS are well-characterized effects of Complex I inhibitor rotenone and Complex III inhibitor antimycin A (AA) [
16]. Interestingly, it was found earlier that salicylate interacted with a Fe-S cluster of mitochondrial Complex I from rat liver which led to generation of ROS [
95]. At the same time, rotenone did not induce significant ROS production in non-photosynthesizing cell suspension cultures of
Rubus fruticosus suggesting that it did not affect the reverse electron transfer [
96]. In contrast to Complex I, Complex III was described as the major source of ROS generation by inactivating the semiquinone radical during the Q cycle after 2.5 mM SA treatments in this cell suspension culture [
96]. Later Nie et al. [
8] demonstrated with fluorescence techniques that 0.5 SA might act directly on the complex III in plant mitochondrial ETC by inhibiting the respiratory activity and causing rapid oxidative burst within minutes in isolated
Arabidopsis mitochondria (). These observations underline the possible organ- and tissue-specific (photosynthetic or not photosynthetic) effects of SA on plant mitochondria. At the same time, the effects of SA on ETC complexes at lower concentration (<0.1 mM) remained unclear in isolated mitochondria of plants. Investigation of the role of SA at normal intercellular concentration is crucial because basal levels of total SA range between 1–10 μg FM
−1 and it is elevated upon infection 10–100 fold higher depending on plant species [
80,
81]. Belt et al. [
9] showed firstly that micromolar concentration of SA increased succinate dehydrogenase (SDH) activity but only when succinate-dependent electron transport was directed through the UQ binding site of SDH, elevating also the succinate:quinone reductase (SQR) activity. In addition, significant mitochondrial ROS production was observed after 7 mM SA treatment which was succinate-dependent using wild type
Arabidopsis and
SDH1-1 (
dsr1) and SDH assembly factor (
sdhaf2) point mutant and knockdown plants [
9]. These excellent articles provided the first quantitative and kinetic evidence for direct involvement of micromolar concentration of SA in an SDH-dependent signaling pathway in
Arabidopsis that contributes to mitochondrial ROS production and leaves to SA-dependent transcriptional regulation.
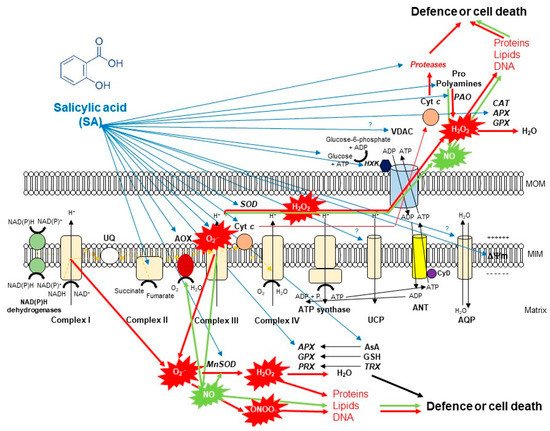
Figure 2. A schematic illustration of the effects of salicylic acid (SA) on the metabolism of reactive oxygen species (ROS) in plant mitochondria. The accumulation of endogenous SA leads to an increase in mitochondrial ROS and nitric oxide (NO) production. Mitochondrial complex III plays the dominant role in superoxide (O2•−) generation upon SA. O2•− is able to react with NO generating peroxynitrite (ONOO−) and thus can regulate the redox status of the cell or initiate cell death. However, the produced O2•− can be converted by superoxide dismutase (SOD) enzyme to H2O2. It was observed that exogenous SA treatments elevated the mitochondrial Mn-SOD activity and gene expression. The decomposition of H2O2 is mediated by several antioxidant enzymes (ascorbate peroxidase, APX; guiacol-peroxidases, POD; peroxiredoxin, PRX) and antioxidants (ascorbate, AsA; glutathione, GSH; thioredoxins, TRX) mediated by SA or H2O2 can exit from mitochondria playing role in cell signaling or oxidizing proteins, lipids and nucleic acids. Moreover, the SA-regulated alternative oxidase (AOX) plays a crucial role in the reduction of mitochondrial ROS and cell death mechanisms. The high concentration of SA not only induces toxic ROS production but also the release of cytochrome c (Cyt c) from the mitochondrial inner membrane through the permeability transition pore (PTP) formed by the voltage-dependent anion channel (VDAC) and the adenine nucleotide transporter (ANT) that contributes to the initiation of cell death. At the same time, mitochondrial hexokinases (HXKs) are important mediators of PTP regulation upon SA. However, the role of many component and molecule of mitochondria and cytosol (e.g., aquaporins, AQP; uncoupling mitochondrial proteins, UCP; polyamines such as spermine, Spm; proline, Pro) exposed to SA is not known in full details. Detailed description and references are in the text.
3. SA-Mediated Defence against Toxic Mitochondrial Oxidative Stress: The Role of Alternative Oxidase (AOX)
The mitigation of toxic ROS levels generated by different cell compartments such as mitochondria is required at each stage of the life cycle. There are various strategies providing a key for high ROS which are mediated by phytohormones such as SA. It can be summarized that the primer source of ROS is originated from the inhibition of mitochondrial ETC. SA by concentration- and time-dependent manner can contribute to the elevated ROS production or scavenging and limiting of ROS in mitochondria of plants (). First of all, the effects of SA on AOX is the most relevant and the most investigated topic. The role of AOX (together with UCP) is crucial when cyt
c is released from mitochondria during the initiation of plant PCD because respiratory electron transport can continue under this circumstance [
117,
118,
119,
120]. In addition, AOX activity can help to decrease ROS production in mitochondria [
121,
122] and contribute to the NO production [
28,
29]. Moreover, changes in the expression of
AOX genes have been proposed to represent an excellent ‘reporter gene’ to evaluate the mitochondrial dysfunction under stress conditions [
104]. Effects of SA on AOX transcripts and protein abundance are highly-researched areas, particularly since the role of SA in thermogenic species
Sauromatum guttatum has been documented [
123]. Later, the induction of AOX by SA has been observed in various plant species such as in tobacco cell suspension culture after 12 h-long-treatment with 1 mM SA [
124], in isolated mitochondria from tobacco leaves within 5 h after 1 mM SA treatment [
125], in
Sauromatum guttatum appendix after 0.01 mM SA treatment [
126], in isolated soybean cell mitochondria after 16 h-long-treatment with 1 mM SA [
94], in isolated tobacco cell mitochondria treated with 0.5 mM SA for 8 h [
118], in
Orobanche seeds exposed to 0.02 mM SA for 1–3 days [
127], in tobacco calli after 8 h-long-treatment with 0.02 mM SA [
128], and in purified mitochondria from the cotyledon of yellow lupine treated with 1 mM SA for 12 h [
129]. It can be concluded that effects of SA on AOX protein level did not depend on the plant cell types (leaf, calli, cell suspension) and SA caused a rapid (within 24 h) changes in the AOX function.
Normann et al. [
7] measured firstly concentration-dependent effects in case of SA treatments in tobacco cell suspension culture. 0.1 mM SA induced an increase in AOX protein levels which correlated with the increase in gene expression of
Aox1 after 4 h. In contrast to this observation, when 0.1 mM SA was applied, the measured increase in AOX was transient and disappeared as SA levels declined in the cells. At the same time, 0.01 mM SA also elevated the expression of other SA-responsive genes (e.g.,
Pathogenesis-related 1,
PR1) but this effect of SA was dependent on active mitochondria [
7]. Matos et al. [
112] also measured that AOX capacity and protein contents increased after 24 h in mitochondria extracted from 1 mM SA-treated soybean seedlings. Interestingly, Authors observed that both
AOX1 and
AOX2b transcripts accumulated in response to SA after 4 h but only
AOX2b expression was significantly higher after 24 h [
112]. These findings suggest also a concentration- and time-dependent effect of SA on the expression kinetics of AOX. This was further confirmed by the research of Cvetkovska and Vanlerberghe [
130]. Surprisingly, the lethal concentration of SA at 3 mM failed to induce the expression of
Aox1a, but 0.1 mM SA elevated
Aox1a transcripts within 4 h and reduced cell death based on the detection of DNA laddering. In contrast to this observation, 0.5 mM SA promoted the accumulation of
Aox1a transcripts after 4 h in tobacco cell suspension culture [
130]. In tobacco leaf, an incompatible plant-bacteria interaction that produced high SA levels and HR was associated with low levels of AOX, whereas an incompatible interaction that produced only low SA levels with defence induction, but no HR, was associated with high levels of AOX [
130]. Several studies about viruses revealed that SA-induced resistance is much simpler in Potato virus X (PVX) execution than SA-induced resistance to Tobacco mosaic virus (TMV) e.g., by mediating ROS [
131]. It was observed that AOX-regulated defensive signaling is the predominant factor in controlling SA-initiated resistance during PVX infection [
132].
Direct effects of SA on the transcriptional regulation of AOX could be an interesting research topic. Based on promoter analysis, the posttranscriptional mechanism of SA in the regulation of
AOX coding sequences via H
2O
2 can take into account in the regulation of mitochondrial stress responses [
52]. Transcript analysis in
Vigna unguiculata leaves has also confirmed that treatment with 0.5 mM SA and 10 mM H
2O
2 induced a certain extent differently the expression of two AOX coding genes,
VuAox1 and
VuAox2b. At the same time, both treatments caused a peak in
VuAox2b expression after 6 h but the effects of SA were more prolonged on this gene [
133]. In addition, NO has been also demonstrated to be an effective inducer of AOX gene expression [
134,
135], which could be also important in SA-mediated stress response. The opposite connection between SA and AOX has been also investigated, but this relationship needs further investigation. The study of Zhang et al. [
136] hints that AOX level may (indirectly with ROS) influence SA level under biotic stress. In transgenic tobacco, which was silenced in the expression of AOX, the piercing-sucking insects (
Empoasca spp.) caused more significant leaf damage. Also, HR-like cell death in response to bacterial infection (
Pseudomonas syringae pv
tomato DC3000) occurred more rapidly in the plants lacking AOX. Interestingly, in both cases, SA levels were significantly higher after hours in the challenged plants lacking AOX than in challenged wild-type plants [
136].