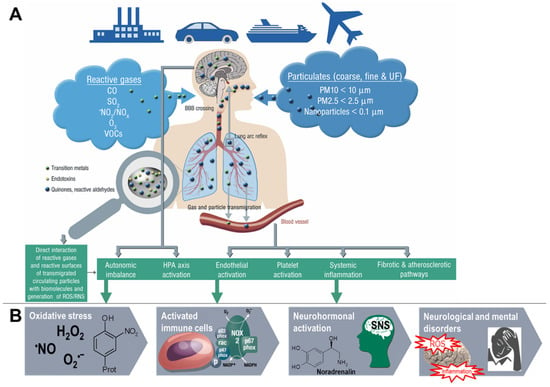
Air pollution is a heterogeneous mixture of various constituents resulting from the complex interaction of multiple emissions and chemical reactions. This mixture comprises solid particles and liquid droplets suspended in the air, i.e., PM2.5, that can include organic carbon (OC), elemental or black carbon (EC), nitrates, sulfates, and metals (e.g., iron, vanadium, nickel, copper, and manganese) as well as gases (e.g., ground level ozone (O3), carbon monoxide (CO), sulfur dioxide (SO2), oxides of nitrogen (NOx)) gaseous organic compounds (e.g., non-methane volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs)), bacterial endotoxins (mostly bound to solid particles or liquid aerosols). There are two possible ways by which air pollutants enter the CNS, either through direct transport of particles into the CNS or via systemic inflammation upon initial recruitment of immune cells in the lung tissue. Once in the organism, the adverse effects of fine particulates on the brain rely mainly on three mechanisms. First, they can induce the release of proinflammatory mediators leading to chronic respiratory and systemic inflammation, thereby affecting the BBB and ultimately triggering neural-immune interaction and resulting in increased production of ROS and chronic oxidative stress. Second, the particles can damage the BBB through the direct formation of ROS and thereby alter the permeability of the barrier. Third, there can be mechanical stimulation of specific mechano-receptors in pulmonary tissue leading to the lung arc reflex and sympathetic activation with the release of vasoconstrictors such as catecholamines.
- Air Pollution
- Pathophysiology
- Oxidative Stress
- Inflammation
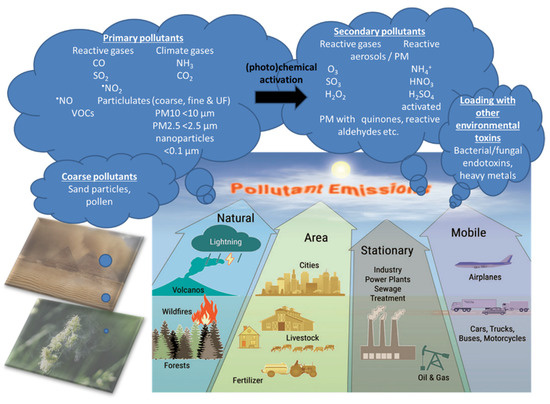
Air Pollution Mixtures and Sources
Pathophysiology of Air-Pollution-Induced Disorders
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. E ects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart. J. 2018, 39, 3543–3550.
- Poschl, U.; Shiraiwa, M. Multiphase chemistry at the atmosphere-biosphere interface influencing climate and public health in the anthropocene. Chem. Rev. 2015, 115, 4440–4475. [Google Scholar] [CrossRef]
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Air Pollution and Climate Change. Available online: https://ec.europa.eu/environ-ment/integration/research/newsalert/pdf/24si_en.pdf (accessed on 4 May 2020).
- Peden, D.B. Pollutants and asthma: Role of air toxics. Environ. Health Perspect. 2002, 110, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, P.E.; Ovrevik, J.; Hetland, R.B.; Becher, R.; Cassee, F.R.; Lag, M.; Lovik, M.; Dybing, E.; Refsnes, M. Importance of size and composition of particles for effects on cells in vitro. Inhal. Toxicol. 2007, 19, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef]
- Munzel, T.; Steven, S.; Frenis, K.; Hahad, O.; Daiber, A. Environmental factors such as noise and air pollution and vascular disease. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report on the Global Tobacco Epidemic, 2017 Monitoring Tobacco Use and Prevention Policies. Available online: https://apps.who.int/iris/bitstream/handle/10665/255874/9789241512824-eng.pdf?sequence=1 (accessed on 4 May 2020).
- Lelieveld, J.; Pozzer, A.; Poschl, U.; Fnais, M.; Haines, A.; Munzel, T. Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Munzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the Air: Air Pollution and Cognitive Dysfunction. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 333–341. [Google Scholar] [CrossRef]
- Wilson, S.J.; Miller, M.R.; Newby, D.E. Effects of Diesel Exhaust on Cardiovascular Function and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 819–836. [Google Scholar] [CrossRef]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012, 2012, 472751. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Solt, A.C.; Henriquez-Roldan, C.; Torres-Jardon, R.; Nuse, B.; Herritt, L.; Villarreal-Calderon, R.; Osnaya, N.; Stone, I.; Garcia, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Heidari Nejad, S.; Takechi, R.; Mullins, B.J.; Giles, C.; Larcombe, A.N.; Bertolatti, D.; Rumchev, K.; Dhaliwal, S.; Mamo, J. The effect of diesel exhaust exposure on blood-brain barrier integrity and function in a murine model. J. Appl. Toxicol. 2015, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, S.; Farbood, Y.; Gharib-Naseri, M.K.; Goudarzi, G.; Rashno, M.; Maleki, H.; Bakhtiari, N.; Nesari, A.; Khoshnam, S.E.; Dianat, M.; et al. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020, 242, 117210. [Google Scholar] [CrossRef]
- Robinson, R.K.; Birrell, M.A.; Adcock, J.J.; Wortley, M.A.; Dubuis, E.D.; Chen, S.; McGilvery, C.M.; Hu, S.; Shaffer, M.S.P.; Bonvini, S.J.; et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 2018, 141, 1074–1084 e1079. [Google Scholar] [CrossRef]
- Perez, C.M.; Hazari, M.S.; Farraj, A.K. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc. Toxicol. 2015, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Diez Roux, A.V.; Castro-Diehl, C.; Cosselman, K.; Golden, S.H.; Hazlehurst, M.F.; Szpiro, A.; Vedal, S.; Kaufman, J.D. The Association between Long-Term Air Pollution and Urinary Catecholamines: Evidence from the Multi-Ethnic Study of Atherosclerosis. Environ. Health Perspect. 2019, 127, 57007. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef]
- D’Angiulli, A. Severe Urban Outdoor Air Pollution and Children’s Structural and Functional Brain Development, From Evidence to Precautionary Strategic Action. Front. Public Health 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S. Planetary Health and the Future of Human Capacity: The Increasing Impact of Planetary Distress on the Human Brain. Challenges 2018, 9, 41. [Google Scholar] [CrossRef]
- Block, M.L.; Calderon-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kroller-Schon, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Vujacic-Mirski, K.; Kuntic, M.; Bayo Jimenez, M.T.; Helmstadter, J.; Steven, S.; et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction-Signatures of the internal exposome. Biofactors 2019, 45, 495–506. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/ijms21124306
