Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Breast milk components contribute to the infant’s immune development and protection, and among other immune factors, immunoglobulins (Igs) are the most studied. The presence of IgA in milk has been known for a long time; however, less information is available about the presence of other Igs such as IgM, IgG, and their subtypes (IgG1, IgG2, IgG3, and IgG4) or even IgE or IgD.
- immunoglobulin
- IgA
- breast milk
- immunoglobulinome
1. Introduction
1.1. Breast Milk: A Source of Immunomodulatory Components
Breast milk is the biological fluid produced by the mother’s breasts of mammalians in order to nourish infants and also to confer on them protection from disease until their own immune system matures [1]. Accordingly, the World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life, followed by continued breastfeeding with adequate complementary foods for up to 2 years or beyond, as mutually desired by mother and infant [2]. Breast milk has been tailored during human evolution to meet the demands of the infant. Its composition varies within feeds, during the day, and between mothers [3]. Interindividual variability has been attributed to genetic variation, maternal adiposity, and nutrition, among other factors [4][5][6]. The composition of human milk is dynamic and changes throughout lactation. The first form of milk produced by the mammary glands during the first 2–4 days after delivery is colostrum, which is produced in low volumes (300–400 mL/day) and has higher levels of protein and lower levels of carbohydrates and fat content than mature breast milk. Moreover, colostrum is richer in immunological components, such as immunoglobulins (Igs), lactoferrin, leucocytes, and oligosaccharides, suggesting that its primary functions are immunological rather than nutritional [3][7]. From days 4–5 after delivery, colostrum changes to transition milk, which is characterized by a higher yield (500–800 mL/day) and by lower protein and Ig content, accompanied by an increase in lactose, fat, and water-soluble vitamins to meet the growth demands. Finally, mature milk remains relatively similar in composition 6 weeks after delivery [3][8]. While 87% of breast milk is water, the remaining 13% is nutritional components and bioactive compounds that have beneficial non-nutritional functions [9]. These latter compounds include a wide range of antimicrobial factors, microorganisms, cytokines, hormones, growth modulators, and digestive enzymes, among others, although the Igs are of special relevance for the baby’s immune protection and development [10].
1.2. The Mammary Gland as a Source of Protective Immunoglobulins for the Newborn
In humans and non-human primates, the transplacental transfer of immunoglobulins (Igs) from the mother to the fetus provides passive immunization to the offspring before delivery. However, it is after delivery when, in many animals, such as rodents or pigs, the Igs present in colostrum, the first breast milk produced, can be absorbed in the small intestine towards the systemic circulation. However, this phenomenon, very well described in pigs and rodents, is rather limited in humans, in which absorption of trace amounts of Ig can be negligible [11][12][13]. From this perspective, the existence of Igs in human breast milk has long been known [14]. The type, structure, and concentration of these Igs differ from those found in plasma [15][16]. Indeed, the Ig composition of breast milk arises from Igs produced locally in the mammary gland or transferred from the plasma (Figure 1).
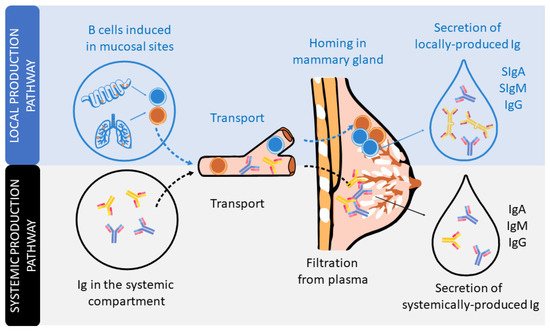
Figure 1. The secretion of Igs in human milk. Schematic figure of the local production pathway of Igs (involving the B cell homing to the mammary gland and participation of the secretory component) and the systemic production pathway (involving the monomeric Igs plasma filtration from plasma).
The dominant Ig in human milk is a special form of IgA, secretory IgA (SIgA), which is common to all mucosal secretions. This particular structure has multiple features and functions that make it optimal for mucosal defense, such as being able to neutralize pathogens before they come into contact with epithelial cells, being highly resistant and stable, and also preventing excessive inflammation or damage to the tissues [17][18][19]. The production of SIgA is induced by pathogens or commensal microorganisms found in mucosal sites after triggering T-helper (Th) and natural killer (NK)-T cells (T-dependent) or innate cells, such as lymphoid cells (ILCs) or plasmacytoid dendritic cells (pDCs) (T-independent). In particular, the switch from IgM+ B lymphocytes to IgA+ B lymphocytes is mainly driven by the transforming growth factor (TGF)-β and cytokines produced by Th2 cells, including interleukin (IL-4), IL-5, IL-6, IL-10, and IL-21 [11][18][20]. It is important to highlight that during the pregnancy period, in order to provide a maternal immune tolerance environment, the ratio of pro- and anti-inflammatory cytokines, related to Th1 and Th2 cells, respectively, is polarized towards a Th2 response. Moreover, this dominance of the Th2 response persists for some months in the neonate, during the lactation period, before reaching the Th1/Th2 equilibrium [21][22][23].
IgA-producing cells in the mammary gland arise from mucosal tissues mainly found in both the gut and airways (Figure 1). The migration of B cells takes place due to their expression of the chemokine receptor (CCR)-10, which binds to the chemokine ligand (CCL)-28 expressed in the mammary gland [24]. IgA is produced in dimers, containing a polypeptide called the J chain, which is excreted by secretory mammary cells. This transport is mediated by the polymeric Ig receptor (pIgR), also termed the secretory component (SC). The pIgR is cleaved after transcytosis and partly remains in the IgA dimer, constituting SIgA antibodies in the breast milk [17]. In addition, breast milk also contains secretory IgM (SIgM), IgM, and IgG antibodies, but in smaller proportions. Like SIgA, there is selective homing to the mammary gland of plasma cells that produce IgM and IgG, which are subsequently transported into breast milk through pIgR. In addition, pIgR can also transport Igs found in serum [15][16].
With regard to functionality, it has been proven that IgA induces tolerance to microbial and food antigens in both mice and human neonates [25][26][27][28]. However, it has also been demonstrated that milk IgG immune complexes are necessary for tolerance induction in mice [29][30]. IgM and IgG—mainly IgG1 and IgG3 in humans [31]—activate the complement pathway for pathogen clearance and initiation of innate response [32]. Commensal-specific IgG and IgA from maternal milk are very important to dampen T-dependent immune responses against commensal microbiota in neonates [33]. With respect to IgG4, it is the least abundant subclass of IgG in human breast milk and serum [34]. However, it increases in allergen response [31] and has anti-inflammatory properties, since it inhibits immune precipitation and complement activation [35]. Therefore, IgG4 is part of the Th2 response [36][37]. IgG2 is well known for having an important role in the defense against bacterial capsular polysaccharide antigens [31] and for its low capacity to activate the complement system [31][36]. It is thought that IgG2 is involved in Th1 response (IgG1 + IgG2 + IgG3), but this is not yet firmly established [36][37]. Moreover, there are studies that report that IgG2, in addition to IgG4, has a low inflammatory potential at intestinal level [38]. IgE is also present in breast milk, but its functions in neonates are still controversial [39]. Furthermore, its levels in childhood seem to depend on maternal IgE concentration [39]. Moreover, allergen-specific IgE and IgG can be transmitted from maternal blood to human breast milk [40].
1.3. Do We Really Know the Immunoglobulin Concentration in Milk?
Many studies have described the Ig levels in breast milk, mainly IgA, but the other Igs have been less studied. The composition of Igs in milk has been addressed from different perspectives, such as population, including geography, genetics, and diet, as well as taking into account different gestational and delivery factors (antibiotics, gestational age, type of delivery), different collection time points (colostrum, transition, and mature milk), and the use of different techniques (ELISA, single radial immunodiffusion, radioimmunoassay, beads). In addition, some of these determinations were performed in a low number of samples. All these factors could explain why IgA concentration in a particular study can differ by up to 50 times from others [41][42][43][44][45]. Thus, comparison among studies is not an easy task. Although some literature exists, less information is available regarding the IgM, IgG, and IgG subclasses, and even less for IgE or IgD, again with the above limitations.
2. Evolution of Studies Quantifying Ig in Breast Milk
To visualize the evolution of the studies over the years, the included articles were separated into periods of time, depending on their publication year, to observe study trends (Figure 2). Overall, since the first studies describing the presence of IgA in breast milk took place in the 1970–1980 period [14][46][47][48][49], a reduced number of studies addressed the quantification of the overall Ig types in the following 30 years. However, a clear increase in the number of articles was found later, specifically those involving the quantification of IgA. This pattern is not followed by IgE or IgD, which are the least studied Igs in breast milk, and only four [50][51][52][53] and two [53][54] articles, respectively, have been found describing the presence of these Igs in breast milk (Figure 2A). It has to be taken into account that although IgA is the most studied Ig type over time and SIgA is the main form of IgA found in breast milk, the majority of articles refer to this Ig type as IgA without specifying whether the IgA quantified was secretory or not. For this reason, in Figure 2A, there is an evolution line for IgA and another for SIgA in addition to the line including both types of IgA. The evolution of the articles describing the IgG subtypes in breast milk was very similar, since they were usually studied together, either in the 80s by ELISA [55] or recently by Luminex assays [41].
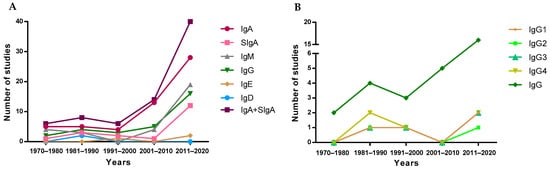
Figure 2. Evolution of the number of studies quantifying Ig types (A) and IgG subtypes (B) over the years.
3. Techniques to Identify and Quantify Ig in Breast Milk
The first studies published in the 70s and 80s describing concentrations of Ig in breast milk used immunodiffusion techniques, such as single radial immunodiffusion. Subsequently, this technique was replaced by enzyme-linked immunosorbent assays (ELISA) and bead-based immunoassays (e.g., Luminex), showing an exponential increase from 2000 onwards (Figure 3). It should be taken into account that the quantification of Ig using these methodologies could introduce an almost twofold variation in levels, thus affecting the absolute concentrations described in the literature due to the methodology used [56]. Turbidimetric and immunonephelometric assays have also been used lately in quick routine analysis. In addition, mass spectrometry has also been used lately for all types of milk protein quantification [57]. Overall, ELISA techniques seem to be preferred due to their sensitivity and potential to particularly target SIgA.
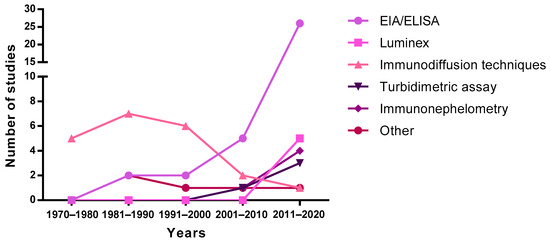
Figure 3. Evolution of the techniques used to measure immunoglobulin levels over the years. EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay.
4. Evolution in the Immunoglobulin Profile during the Lactation Period: The Breast Milk Immunoglobulinome
When we study the complete set of metabolites in a cell, tissue, organ, or organism, we refer to them as metabolome; when the attention is focused on the set of expressed proteins, we call it proteome; and if we concentrate our attention on the set of all RNA transcripts, including coding and noncoding, in an individual or a population of cells, we call it transcriptome. Thus, the set of Igs present in a particular fluid or organic compartment could be referred to as immunoglobulinome. Overall, this immunoglobulinome should also be established at a given time and under defined conditions.
In line with this, breast milk is a dynamic fluid whose levels and proportions of Igs change during lactation. This characteristic profile, then, is different at each stage: colostrum, transition, and mature milk. The overall pool of Igs in breast milk includes not only IgA, but also, in lower proportions, the other Ig classes (IgM, IgG, IgE, and IgD), and more recently, the subclasses of IgG have also been studied. Overall, and taking into account the previous considerations, to refer to this particular mixture of Igs at any specific period, in this review we will use the term “breast milk (BM) immunoglobulinome”.
After considering all the articles published referring to Ig composition in breast milk using the criteria established in the Material and Methods section, the data have been compiled and organized in different tables according to the type of Ig. The tables include the critical aspects determining the Ig concentration described: type of milk (collection day or period), main population characteristics (number of samples analyzed, location, etc.), and finally, the method used for its analysis. All these factors can have an influence in the final concentration described. Studies involving colostrum were considered from d1–d5, the transition period from d6–d15, and from then on mature milk. The mean values from each study, independently of the number of samples they are derived from, have also been compiled and expressed together in Figure 4.
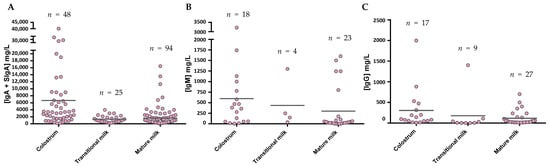
Figure 4. IgA (A), IgM (B), and IgG (C) levels presented in the literature throughout the different phases of breastfeeding. The mean values from each Ig were calculated and shown in the graph using the values provided in the articles for a particular group, independently of the number of samples they are derived from. Figure A takes into account both the determinations obtained from IgA studies and those that claim to measure SIgA specifically.
The levels of IgA (Figure 4A), as described in the individual studies evaluating this concentration in different stages of lactation [58][59][60][61][62][63][64], displayed the highest values in colostrum (~7500 mg/L), with lower levels in transition and mature milk (~1600–2000 mg/L). Due to the variability among studies, no clear differences between transition and mature milk IgA content can be observed. The number of reports studying IgM content in breast milk is much lower than those evaluating IgA, and very few focused on the transition period (Figure 4B). However, a decreasing tendency in IgM content can be observed from colostrum (~600 mg/L) to transition milk (~430 mg/L) and finally to mature milk (~260 mg/L). With regard to IgG, since this is the least studied Ig in breast milk, the results shown here come from a very few studies (Figure 4C), and the overall results are influenced by particular studies with very high values (>800 mg/L). In any case, their levels amount to 180–1100 mg/L. IgE and IgD are minimal in the BM immunoglobulinome at any stage studied, and very few studies have found their presence, as will be further discussed later.
The great variability in terms of Ig concentration makes it difficult to compile results and draw conclusions; thus, their relative proportion may help to make the data more comparable among studies. However, very few studies reported all three levels of IgA, IgM, and IgG [41][45][51][65][66]. Thus, an overall distribution of Ig proportions was calculated on the basis of the mean values obtained for all values and is shown in Figure 5.
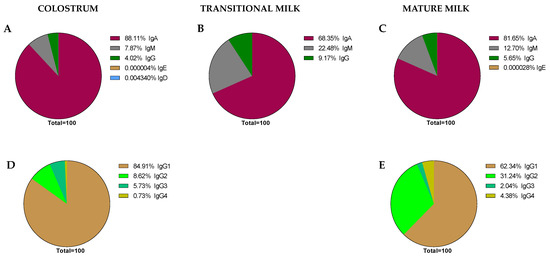
Figure 5. Global proportions from the immunoglobulin concentrations found in the literature. Proportions of Ig classes are expressed in each stage of lactation: colostrum (A), transition (B), and mature (C) milk. IgG subclass proportions were expressed in colostrum (D) and mature milk (E), as there are no current data for transition milk in this regard.
As expected, it can be observed that IgA is the predominant Ig in the BM immunoglobulinome at any stage of lactation; however, based on our calculations, it seems that the relative proportion of IgA is higher in colostrum (~88.11%) than in transition or mature milk (~68.35–81.65%). It is interesting, though, that the lower proportion in these two last stages of lactation seems to be due to a higher proportion of IgM (~22.45–12.70% vs. ~7.87% in colostrum) in the transition and mature periods. However, these proportions, as noted before, are calculations derived from the current values found in the literature and may not reflect the real BM immunoglobulinome, which can only be derived after having real data from independent studies taking into account all types of Ig in the same sample and at different collection time points.
Aside from some old studies dating mostly from the 80s [55][67], only in the last 10 years, and due to the use of the Luminex techniques, have the studies on the BM immunoglobulinome addressed the IgG subtypes in more depth [41][51]. In this case, the proportion of IgG1, IgG2, IgG3, and IgG4, the main human isotypes [31], have been described in colostrum (Figure 5D) and mature milk (Figure 5E). However, there are no available data on IgG isotypes during the transition period. Regarding their relative proportions, the IgG1 percentage is the highest, followed by IgG2, IgG3, and IgG4. This particular composition, with a predominance of the Th1 response (IgG1 + IgG2 + IgG3) over the Th2 response (IgG4), suggests the breast milk regulatory activity on the neonatal Th1/Th2 balance to minimize the Th2 environment that predominates in the intrauterine space [38][68]. The ratio between these IgG can be of importance in observational studies evaluating the factors influencing breast milk immune composition. A certain diet or particular situations (delivery type or length of gestation period) may lead to changes in this balance that deserve to be studied in depth in the future.
This entry is adapted from the peer-reviewed paper 10.3390/nu13061810
References
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635.
- World Health Organization. 10 Facts on Breastfeeding. Available online: (accessed on 1 January 2021).
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74.
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 0048.
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662.
- De la Garza Puentes, A.; Martí Alemany, A.; Chisguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero, M.; Segura, M.T.; Campoy, C.; et al. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The Preobe Study. Nutrients 2019, 11, 2154.
- Sriraman, N.K. The Nuts and Bolts of Breastfeeding: Anatomy and Physiology of Lactation. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 305–310.
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584.
- Picciano, M.F. Nutrient composition of human milk. Pediatr. Clin. N. Am. 2001, 48, 53–67.
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279.
- Telemo, E.; Hanson, L.A. Antibodies in milk. J. Mammary Gland Biol. Neoplasia 1996, 1, 243–249.
- Van De Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376.
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153.
- Brandtzaeg, P. Human Secretory Immunoglobulins. 4. Quantitation of Free Secretory Piece. Acta Pathol. Microbiol. Scand. 1971, 79, 189–203.
- Larson, B.L.; Heary, H.L., Jr.; Devery, J.E. Immunoglobulin Production and Transport by the Mammary Gland. J. Dairy Sci. 1980, 63, 665–671.
- Matson, A.P.; Thrall, R.S.; Rafti, E.; Lingenheld, E.G.; Puddington, L. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin. Mol. Allergy 2010, 8, 9.
- Brandtzaeg, P. The Mucosal Immune System and Its Integration with the Mammary Glands. J. Pediatr. 2010, 156, S8.
- Brandtzaeg, P. Secretory IgA: Designed for anti-microbial defense. Front. Immunol. 2013, 4, 222.
- Cerutti, A.; Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 2008, 28, 740–750.
- Boyaka, P.N. Inducing mucosal IgA: A challenge for vaccine adjuvants and delivery systems. J. Immunol. 2017, 199, 9–16.
- McFadden, J.P.; Thyssen, J.P.; Basketter, D.A.; Puangpet, P.; Kimber, I. T helper cell 2 immune skewing in pregnancy/early life: Chemical exposure and the development of atopic disease and allergy. Br. J. Dermatol. 2015, 172, 584–591.
- Kuroda, K.; Nakagawa, K.; Horikawa, T.; Moriyama, A.; Ojiro, Y.; Takamizawa, S.; Ochiai, A.; Matsumura, Y.; Ikemoto, Y.; Yamaguchi, K.; et al. Increasing number of implantation failures and pregnancy losses associated with elevated Th1/Th2 cell ratio. Am. J. Reprod. Immunol. 2021, e13429.
- Rio-Aige, K.; Azagra-Boronat, I.; Massot-Cladera, M.; Selma-Royo, M.; Parra-Llorca, A.; González, S.; García-Mantrana, I.; Castell, M.; Rodríguez-Lagunas, M.J.; Collado, M.C.; et al. Association of maternal microbiota and diet in cord blood cytokine and immunoglobulin profiles. Int. J. Mol. Sci. 2021, 22, 1778.
- Wilson, E.; Butcher, E.C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J. Exp. Med. 2004, 200, 805–809.
- Favre, L.; Spertini, F.; Corthésy, B. Secretory IgA Possesses Intrinsic Modulatory Properties Stimulating Mucosal and Systemic Immune Responses. J. Immunol. 2005, 175, 2793–2800.
- Sletten, G.B.G.; Halvorsen, R.; Egaas, E.; Halstensen, T.S. Casein-specific immunoglobulins in cow’s milk allergic patient subgroups reveal a shift to IgA dominance in tolerant patients. Pediatr. Allergy Immunol. 2007, 18, 71–80.
- Gloudemans, A.K.; Lambrecht, B.N.; Smits, H.H. Potential of Immunoglobulin A to Prevent Allergic Asthma. Clin. Dev. Immunol. 2013, 2013, 542091.
- Verhasselt, V. Neonatal tolerance under breastfeeding influence. Curr. Opin. Immunol. 2010, 22, 623–630.
- Mosconi, E.; Rekima, A.; Seitz-Polski, B.; Kanda, A.; Fleury, S.; Tissandie, E.; Monteiro, R.; Dombrowicz, D.D.; Julia, V.; Glaichenhaus, N.; et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010, 3, 5.
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen- specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113.
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520.
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786.
- Koch, M.A.; Reiner, G.L.; Lugo, K.A.; Kreuk, L.S.M.; Stanbery, A.G.; Ansaldo, E.; Seher, T.D.; Ludington, W.B.; Barton, G.M. Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life. Cell 2016, 165, 827–841.
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186.
- van der Zee, J.S.; van Swieten, P.; Aalberse, R.C. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 1986, 64, 415–422.
- Hjelholt, A.; Christiansen, G.; Sørensen, U.S.; Birkelund, S. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog. Dis. 2013, 67, 206–213.
- Holdsworth, S.R.; Kitching, A.R.; Tipping, P.G. Th1 and Th2T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999, 55, 1198–1216.
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020.
- Wright, A.L.; Sherrill, D.; Holberg, C.J.; Halonen, M.; Martinez, F.D. Breast-feeding, maternal IgE, and total serum IgE in childhood. J. Allergy Clin. Immunol. 1999, 104, 589–594.
- Hochwallner, H.; Alm, J.; Lupinek, C.; Johansson, C.; Mie, A.; Scheynius, A.; Valenta, R. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J. Allergy Clin. Immunol. 2014, 134, 1213–1215.
- Moles, L.; Manzano, S.; Fernández, L.; Montilla, A.; Corzo, N.; Ares, S.; Rodríguez, J.M.; Espinosa-Martos, I. Bacteriological, biochemical, and immunological properties of colostrum and mature milk from mothers of extremely preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 120–126.
- Striker, G.A.J.; Casanova, L.D.; Nagao, A.T. Influence of type of delivery on A, G and M immunoglobulin concentration in maternal colostrum. J. Pediatr. 2004, 80, 123–128.
- Chantry, C.J.; Israel-Ballard, K.; Moldoveanu, Z.; Peerson, J.; Coutsoudis, A.; Sibeko, L.; Abrams, B. Effect of flash-heat treatment on immunoglobulins in breast milk. J. Acquir. Immune Defic. Syndr. 2009, 51, 264–267.
- Nathavitharana, K.A.; Catty, D.; McNeish, A.S. IgA antibodies in human milk: Epidemiological markers of previous infections? Arch. Dis. Child. 1994, 71, 192–197.
- Mickleson, K.N.P.; Moriarty, K.M. Immunoglobulin levels in human colostrum and milk. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 381–384.
- Whyatt, R.G.; Garcia, B.; Cáceres, A.; Mata, L.J. Immunoglobulins and antibodies in colostrum and milk of Guatemalan mayan women. Arch. Latinoam. Nutr. 1972, 22, 4.
- Reddy, V.; Bhaskaram, C.; Raghuramulu, N.; Jagadeesan, V. Antimicrobial Factors in Human Milk. Acta Pædiatrica 1977, 66, 229–232.
- Ford, J.E.; Law, B.A.; Marshall, V.M.E.; Reiter, B. Influence of the heat treatment of human milk on some of its protective constituents. J. Pediatr. 1977, 90, 29–35.
- Önes, S.U. Immunoglobulins of human colostrum and milk. J. Pediatr. 1978, 94, 497–498.
- Duchén, K.; Björkstén, B. Total IgE levels in human colostrum. Pediatr. Allergy Immunol. 1996, 7, 44–47.
- Espinosa-Martos, I.; Montilla, A.; De Segura, A.G.; Escuder, D.; Bustos, G.; Pallás, C.; Rodríguez, J.M.; Corzo, N.; Fernández, L. Bacteriological, biochemical, and immunological modifications in human colostrum after holder pasteurisation. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 560–568.
- Meng, X.; Dunsmore, G.; Koleva, P.; Elloumi, Y.; Wu, R.Y.; Sutton, R.T.; Ambrosio, L.; Hotte, N.; Nguyen, V.; Madsen, K.L.; et al. The Profile of Human Milk Metabolome, Cytokines, and Antibodies in Inflammatory Bowel Diseases Versus Healthy Mothers, and Potential Impact on the Newborn. J. Crohn’s Colitis 2018, 13, 431–441.
- Bahna, S.L.; Keller, M.A.; Heiner, D.C. IgE and IgD in human colostrum and plasma. Pediatr. Res. 1982, 16, 604–607.
- Keller, M.A.; Heiner, D.C.; Myers, A.S.; Reisinger, D.M. IgD in human colostrum. Pediatr. Res. 1985, 19, 122–126.
- Mehta, P.D.; Mehta, S.P.; Isaacs, C.E. Distribution of IgG subclasses in human colostrum and milk. Immunol. Lett. 1989, 22, 235–238.
- Dunn, A.; Duffy, C.; Gordon, A.; Morrison, S.; Argűello, A.; Welsh, M.; Earley, B. Comparison of single radial immunodiffusion and ELISA for the quantification of immunoglobulin G in bovine colostrum, milk and calf sera. J. Appl. Anim. Res. 2018, 46, 758–765.
- Klein, L.D.; Huang, J.; Quinn, E.; Martin, M.A.; Breakey, A.A.; Gurven, M.; Kaplan, H.; Valeggia, C.; Jasienska, G.; Scelza, B.; et al. Variation among populations in the immune protein composition of mother’s milk reflects subsistence pattern. Evol. Med. Public Health 2018, 230–245.
- Gross, S.J.; Buckley, R.H.; Wakil, S.S.; McAllister, D.C.; David, R.J.; Faix, R.G. Elevated IgA concentration in milk produced by mothers delivered of preterm infants. J. Pediatr. 1981, 99, 389–393.
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. J. Nutr. 2011, 141, 1181–1187.
- Ronayne de Ferrer, P.A.; Slobodianik, N.H.; Lopez, N.; Sambucetti, M.E.; Sanahuja, J.C. Immunoglobulin A level in human milk from mothers delivering preterm. Am. J. Clin. Nutr. 1984, 40, 465–467.
- Jatsyk, G.V.; Kuvaeva, I.B.; Gribakin, S.G. Immunological Protection of the Neonatal Gastrointestinal Tract: The Importance of Breast Feeding. Acta Pædiatrica 1985, 74, 246–249.
- Weaver, L.T.; Arthur, H.M.L.; Bunn, J.E.G.; Thomas, J.E. Human milk IgA concentrations during the first year of lactation. Arch. Dis. Child. 1998, 78, 235–239.
- Trégoat, V.; Montagne, P.; Béné, M.C.; Faure, G. Increases of Iga milk concentrations correlate with IgA2 increment. J. Clin. Lab. Anal. 2001, 15, 55–58.
- Palmeira, P.; Costa-Carvalho, B.T.; Arslanian, C.; Pontes, G.N.; Nagao, A.T.; Carneiro-Sampaio, M.M.S. Transfer of antibodies across the placenta and in breast milk from mothers on intravenous immunoglobulin. Pediatr. Allergy Immunol. 2009, 20, 528–535.
- Koenig, Á.; de Albuquerque Diniz, E.M.; Correia Barbosa, S.F.; Costa Vaz, F.A. Immunologic factors in human milk: The effects of gestational age and pasteurization. J. Hum. Lact. 2005, 21, 439–443.
- Ruiz, L.; Espinosa-Martos, I.; García-Carral, C.; Manzano, S.; McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; et al. What’s normal? Immune profiling of human milk from healthy women living in different geographical and socioeconomic settings. Front. Immunol. 2017, 8, 696.
- Keller, M.A.; Heiner, C.; Kidd, R.M.; Myers, A.S. Local production of IgG4 in human colostrum. J. Immunol. 1983, 130, 1654–1657.
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228.
This entry is offline, you can click here to edit this entry!
