Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The presented work focuses on the application of spectroscopic methods, such as Infrared Spectroscopy (IR), Fourier Transform Infrared Spectroscopy (FT-IR), Raman spectroscopy, Ultraviolet and Visible Spectroscopy (UV-Vis), X-ray spectroscopy, and Mass Spectrometry (MS), which are widely employed in the investigation of the surface properties of dental materials.
- dental materials
- dental ceramics
- spectroscopy
- IR
- FT-IR
- Raman spectroscopy
- UV-Vis
- X-ray spectroscopy
- XRF
- XRD
- MS
1. Introduction
For centuries in dentistry, the search remains for the best methods and materials to restore missing tooth structures or replace missing teeth [1][2][3]. Modern dentistry, in addition to its primary role to improve the health of masticatory system, increasingly focuses on the aesthetics of the preformed reconstructions [4][5][6][7]. The rapid development of this field is possible thanks to the constant introduction of new materials and research techniques [8][9][10][11][12][13][14]. Due to wide range of (ceramic, metallic, synthetic or composite) dental materials available on the market, it is crucial that dental technicians and/or dentists choose the appropriate method by taking into account its limitations and features [13][15][16][17][18][19][20].
The application of the material in the oral cavity, i.e., in direct contact with tissues, places special demands on this group of biomaterials regarding their physicochemical and biological properties, i.e., biocompatibility, local and general harmlessness to the organism [18][21][22], resistance to the effects of physicochemical factors in the oral cavity [21] and biophysical indifference [18][19]. In addition, the accuracy in mimicking natural tooth shapes [19], stability of mechanical properties [19][22], ease processing [21][23], appropriate aesthetics [19][24], and finally a moderate price [19][23] are further requirements that need to be met. The first phenomenon that occurs after the introduction of the biomaterial into the oral cavity is the formation of a biofilm on its surface [25][26][27]. In order to prevent the micro-leakage and the biofilm formation, it is important to know the material structural and chemical components [7][28][29]. Surface structure, roughness, chemical composition and reactivity are just a few of the main characteristics that should be assessed before qualifying particular material for dental applications [18][19][27][30][31]. In addition, surface characteristics are related to other properties of the material, such as mechanical or chemical features, enabling detailed understanding how the material behaves under oral conditions [32][33].
The advanced development of microscopic and spectroscopic techniques enables a detail study of the chemical structure and surface phenomena [20][34] of studied materials. Microscopy enables observation of surfaces, and structural and intra-material changes at high magnification [35][36][37]. It includes, inter alia, Optical Microscopy [38][39][40], Electron Microscopy [39][40][41][42], X-ray Microscopy [39][40], and Scanning Probe Microscopy [39][41].
These techniques allow for advanced experimental research, which in combination with powerful acquisition procedures and data processing, enable detailed analysis of the studied system. Besides morphological and structural characterizations, microscopic techniques provide additionally information on the quantitative and qualitative chemical composition of the analysed sample either at a selected point, or along a given line, thus providing elemental maps (Energy Dispersive X-ray Spectroscopy—EDS), crystal orientation (Electron Backscatter Diffraction—EBSD), and many other characteristics of dental biomaterials [40][43][44][45]. The use of microscopic methods for material testing is widely described in the literature; therefore, in this paper we present the application of spectroscopic techniques, basic principles of operation, their advantages and limitations, as well as their application for studying dental materials.
Spectroscopy covers a lot of techniques based on the interaction of the electromagnetic radiation with the matter; sometimes, the term spectroscopy refers to an analytical technique involving generation and interpretation of spectra. The use of spectroscopy, especially in combination with microscopic techniques, provides valuable information on the chemical structure of dental materials. The major advantages of spectroscopic techniques are seen in the fact that they are non-destructive and using small amount of sample weights [34][46][47][48][49][50][51][52]. Spectroscopic techniques including: Infrared spectroscopy (IR), Fourier Transform Infrared Spectroscopy (FT-IR), Raman spectroscopy, Ultraviolet and Visible spectroscopy (UV-Vis), X-ray spectroscopy, and Mass Spectrometry (MS) are very useful in the dental material studies [53].
2. Fundamentals and Division of Spectroscopy
For the purpose of a comprehensive overview of spectroscopic techniques, it is necessary to apply various criteria for classification of the techniques. Most often, the following classification criteria are adopted: the range of electromagnetic radiation, properties of the studied systems, and the method of collecting a spectrum, referring to the way of energy exchange between the radiation and the matter. The division of spectroscopy according to the radiation range is actually related to various experimental techniques as listed in Table 1. Depending on the radiation range, different radiation sources, detectors and dispersion devices are used. In optical spectroscopy, prisms and diffraction gratings are most frequently used as dispersion devices [54][55][56][57].
Table 1. Spectroscopic techniques in different ranges of electromagnetic spectrum radiation.
| Region of Electromagnetic Spectrum | Wavelength Range λ (m) | Spectroscopic Technique |
|---|---|---|
| Microwave | 1–10−3 | Microwave spectroscopy |
| Infrared | 10−3–10−6 | Infrared spectroscopy Raman spectroscopy |
| Ultraviolet and visible | 10−6–10−8 | UV-Visible spectroscopy Atomic absorption spectroscopy Fluorescence spectroscopy Phosphorescence spectroscopy |
| X-ray | 10−9–10−12 | X-ray diffraction X-ray fluorescence X-ray photoelectron spectrometry Mass spectrometry |
| γ-ray | 10−12–10−14 | Mossbauer spectroscopy |
Spectroscopy can be analysed based on the intrinsic aspects of the studied process. In this regard, the following types of spectroscopy can be differentiated: nuclear spectroscopy, atomic spectroscopy, and molecular spectroscopy with particular emphasis on the spectroscopy of condensed systems. Such division is related to the specific energy levels taking part in the energetic transition of the studied system. Each type of spectroscopy is associated with specific motion of the constituents of the system at a microscopic level, and thus differs in the magnitude of the energy between transition energetic states [34][53][58][59].
Depending on the radiation measurement process, one distinguishes three types of spectra: absorption, emission and Raman spectroscopy, respectively. Absorption spectra can be defined as a set of all transitions from lower to higher levels so, they correspond to an increase of the system energy (photon uptake). The simplest type of absorption spectrum arises when the lowest energy level, i.e., the basic level, is occupied. The occupation of energy levels is related to the thermodynamic equilibrium, which is determined by the temperature of the system. It is assumed that only the basic level is filled at room temperature. Emission spectra can be defined as a set of all transitions from higher to lower energy levels. Transitions in emission spectra correspond to the reduction of the energy, i.e., the radiation of photons. The characteristic feature of Raman spectra is the change in the frequency of the scattered radiation (νr) in relation to the frequency of the incident radiation (νp) [60][61].
3. Infrared Spectroscopy (IR) and Fourier Transform Infrared Spectroscopy (FT-IR)
3.1. Principle of the Technique
The area of the electromagnetic spectrum with the wavenumber (reciprocal of the wavelength) from approx. 14,000 to 200 cm−1, i.e., between the visible and the microwave region, is called the infrared (IR). The absorption of such energy amount within this region is large enough to cause the chemical bonds to oscillate, but not enough to cause their breakage. Molecules rotate around their symmetry axes and at the same time their atoms oscillate between equilibrium positions. The IR absorption spectra are obtained by measuring the relative intensity of the transmitted or absorbed radiation as a function of the wavenumber, and they are presented in form of a plot of the transmittance or absorbance of the radiation versus the wavenumber (cm−1).
There are many types of IR spectrophotometers, e.g., filter photometers, double-beam spectrophotometer, Fourier transform spectrometer, attenuated total reflectance (ATR) FT-IR instrument [55]. In modern Fourier transform devices, which consists of illuminating simultaneously the sample with a beam of radiation from the entire tested IR range. After this beam has passed through the sample, the beam from the same source has not been interfered with, and the spectrum is obtained using the Fourier transform of the recorded interference spectrum. This requires the use of equipment with a software that performs this mathematical operation and provides information about vibrations in the form of an interferogram. FT-IR spectroscopy allows to determine the characteristic vibrations of atomic groups of the studied compound.
Due to the fact that the FT-IR spectrum is characteristic for a given substance, infrared spectrophotometric analysis is most often used for qualitative analysis. In addition to characterizing a pure substance by means of FT-IR analysis, the presence of additional substances in a studied mixture, as well as interactions between atomic groups of different compounds in the mixture can be examined. Changes in the spectrum (presence of additional peaks) indicate the presence of other functional groups (presence of another compound), while the shifting of the peaks relative to the spectrum of the original pure compound indicates interactions of a given atomic group with other groups of the studied mixture [61][62]. Besides, the following infrared spectroscopic techniques can be currently differentiated: transmission spectroscopy (TS), internal reflection spectroscopy (IRS), which is also referred to as attenuated total reflection (ATR), mirror reflection (ERS—external reflection spectroscopy), diffuse reflection spectroscopy (DRS), emission spectroscopy (ES) and photoacoustic spectroscopy (PAS) [63][64].
3.2. Type of Tested Samples
In infrared spectroscopy substances can be examined in a gaseous, liquid and solid state. Gas-sample spectra can be obtained by inserting the gaseous sample into a purged cuvette. Liquids can be tested in a pure form, or in a solution form. Liquid samples are placed between the sodium chloride plates; approx. One to ten milligrams of a liquid substance is needed. For liquids that dissolve sodium chloride, silver chloride plates can be used instead. Solid samples are typically tested in a form of suspension, pellet, or deposited glassy film (a solid film on a glass substrate). The spectrum of a solid sample most frequently is obtained by dispersing the sample within an alkali halide pellet. The spectra of solids can also be obtained by preparing a film of the solid sample, following evaporation of the solvent from a droplet deposited on a sodium chloride plate of a solution containing the studied compound [55][65].
3.3. Sample Characteristics
FT-IR spectroscopy allows to determine the characteristic vibrations for groups present in a compound. Due to the fact that the FT-IR spectrum is a spectrum characteristic for a given substance, infrared spectrophotometric analysis is most often used for qualitative research. In addition to distinguishing between pure substances by means of FT-IR analysis, it is possible to investigate the presence of additional substances as well as their interactions with individual groups of the original compound. Changes in the spectrum (presence of additional peaks) indicate the presence of other functional groups (presence of another compound), while shifts in the directions of other wavelengths than for the spectrum of the original sample indicate the interaction of a given group with a different group of the added substance [61][62][66].
For example, the FT-IR method was used to identify the structure of a dental material, i.e., fibroin thanks to application of FT-IR, a NH functional group in fibroin was recognised as an amino acid structure with a peak of 3309.80 cm−1 [67]. Measurement of absorbance using FT-IR led to a spectra of the various bioceramic powders and finally their identification [68].
3.4. Advantages and Limitations
Currently, infrared spectroscopy is widely used technique to identify functional groups and chemical compounds (both organic and inorganic), as well as to assess the purity of a compound. It is inexpensive, instruments are easy to be operated, and IR spectra are obtained quickly [55]. Infrared spectroscopy is a promising alternative to other techniques, as it is not time consuming with respect to sample preparation, measurement and interpretation of the results; it is also non-invasive and relatively simple measuring technique. As all frequencies are measured simultaneously in FT-IR spectroscopy, the spectrum is obtained for a few seconds, being advantageous in comparison with conventional infrared spectroscopy measurements lasting [34][69][70][71]. The undoubted advantage of infrared spectroscopy techniques is the ability to test small amounts of the material [62], and in ATR technique, the ability to penetrate of the light beam an into a sample depth of about 0.5–3 µm [72].
The limitation of IR is the fact that in classic spectrometers, the IR spectra are obtained by examining the absorption of a specific beam of monochromatic radiation (one wavelength), and then sweeping the sample by gradually changing the wavelength during measurement with a dispersion element (prism, diffraction grating). Before the measurement, it is often necessary to properly prepare the sample, e.g., grinding the test material, thoroughly mixing with the potassium bromide (KBr) matrix and compressing under pressure to form a tablet. The sample should not contain water, because water strongly absorbs radiation with the wavenumber from approximately 3700 cm−1 and 1630 cm−1 (this absorption may obscure the bands of the tested substance) [55].
In the ATR sampling system, the main source of errors during the measurement is the imperfect contact between the sample and the diamond crystal [65]. The next drawback of FT-IR instruments is the fact that they are equipped only with a single beam, whereas dispersive instruments generally have a double beam [69].
3.5. Applications
IR spectroscopy was used to track the polymerization kinetics of dental resins [73][74] and adhesives [75][76][77][78] to improve the mechanical features of the dental material. The IR technique was also used to determine the role of intermolecular collagen cross-linking in the mechanical behaviour of dentin [79], to evaluate the structure of heterocyclic compounds as candidates for pulp regeneration [80] and to analyse new generation biomimetic materials replicating the mineral organic dentin and enamel complex [81].
The FT-IR technique was used for the structural characterization of dental materials such as: implant materials [82], biopolymers [83], ceramics [84], resin nanocomposites [85], implant coatings [86][87][88], bioceramics [89], resins [90][91][92][93][94], cements [95], bioglass [96], and self-curing materials [97].
3.6. Spectrum Example
Figure 1 shows the FT-IR spectrum of self-curing polymethyl methacrylate (PMMA)-based dental materials (UNIFAST III, GC Corporation, Tokyo, Japan) control samples prepared by mixing UNIFAST III resin powder (GC Corporation, Tokyo, Japan) with UNIFAST liquid monomer (GC Corporation, Tokyo, Japan). The powder spectrum of GC UNIFAST III is depicted in the inset of Figure 1. The band at around 1132 cm−1 is the characteristic absorption vibration of PMMA. The bands at about 1218 cm−1, 1361cm−1, 1735 cm−1 and 2927 cm−1 are assigned to υ(C–O) stretching vibration, wagging vibration of C–H, C=O stretching, and C–H stretching, respectively [97].
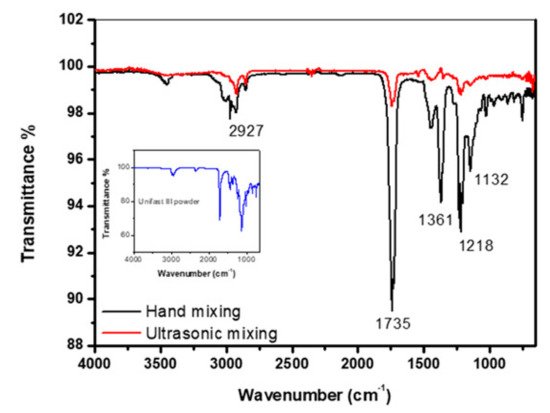
Figure 1. FT-IR spectrum of control specimen made by hand and ultrasonic mixing methods as well as the spectrum corresponding to UNIFAST III powder [97].
Figure 2 shows the FT-IR data of samples prepared by mixing UNIFAST III (GC Corporation, Tokyo, Japan) resin powder with ultrasonic-mixed UNIFAST (GC Corporation, Tokyo, Japan) liquid monomer, which includes starting materials and reinforced, nanosized hexagonal boron nitride h-BN (US Research Nanomaterials Co., Ltd., Houston, TX, USA) at various concentrations. Most researchers agree that there are two distinct IR absorption bands in boron nitride films. These are the band around 1380 cm−1 (in plane) and the band around 780 cm−1 (out of plane), which is due to B–N stretching and B–N–B bending, respectively [97].
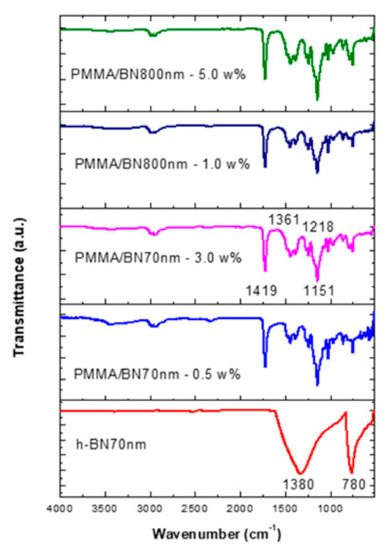
Figure 2. FT-IR data of specimens made by ultrasonic mixing for nano-sized h-BN reinforcement with different concentrations [97].
This entry is adapted from the peer-reviewed paper 10.3390/ma14102624
References
- Alzahrani, K.M. Implant Bio-mechanics for Successful Implant Therapy: A Systematic Review. J. Int. Soc. Prev. Commun. Dent. 2020, 10, 700–714.
- Yu, S.H.; Hao, J.; Fretwurst, T.; Liu, M.; Kostenuik, P.; Giannobile, W.V.; Jin, Q. Sclerostin-Neutralizing Antibody Enhances Bone Regeneration Around Oral Implants. Tissue Eng. Part A 2018, 24, 1672–1679.
- Beltramini, G.; Russillo, A.; Baserga, C.; Pellati, A.; Piva, A.; Candotto, V.; Bolzoni, A.; Beltramini, G.; Rossi, D.; Bolzoni, A.; et al. Collagenated heterologous cortico-cancelleus bone mix stimulated dental pulp derived stem cells. J. Biol. Regul. Homeost. Agents 2020, 34, 1–5.
- Jiang, X.; Cao, Z.; Yao, Y.; Zhao, Z.; Liao, W. Aesthetic evaluation of the labiolingual position of maxillary lateral incisors by orthodontists and laypersons. BMC Oral Health 2021, 21, 42.
- Gomez-Meda, R.; Esquivel, J.; Blatz, M.B. The esthetic biological contour concept for implant restoration emergence profile design. J. Esthet. Restor. Dent. 2021, 33, 173–184.
- Furukawa, M.; Wang, J.; Kurosawa, M.; Ogiso, N.; Shikama, Y.; Kanekura, T.; Matsushita, K. Effect of green propolis extracts on experimental aged gingival irritation in vivo and in vitro. J. Oral Biosci. 2021, 63, 58–65.
- Koutouzis, T. Implant-abutment connection as contributing factor to peri-implant diseases. Periodontology 2000 2019, 81, 152–166.
- Birch, S.; Ahern, S.; Brocklehurst, P.; Chikte, U.; Gallagher, J.; Listl, S.; Lalloo, R.; O’Malley, L.; Rigby, J.; Tickle, M.; et al. Planning the oral health workforce: Time for innovation. Commun. Dent. Oral Epidemiol. 2021, 49, 17–22.
- Joda, T.; Yeung, A.W.K.; Hung, K.; Zitzmann, N.U.; Bornstein, M.M. Disruptive Innovation in Dentistry: What It Is and What Could Be Next. J. Dent. Res. 2020, 100, 448–453.
- Joda, T.; Bornstein, M.M.; Jung, R.E.; Ferrari, M.; Waltimo, T.; Zitzmann, N.U. Recent trends and future direction of dental research in the digital era. Int. J. Environ. Res. Public Health 2020, 17, 1987.
- Bastos, N.A.; Bitencourt, S.B.; Martins, E.A.; De Souza, G.M. Review of nano-technology applications in resin-based restorative materials. J. Esthet. Restor. Dent. 2020.
- Glied, A.; Mundiya, J. Implant Material Sciences. Dent. Clin. N. Am. 2021, 65, 81–88.
- Saeidi Pour, R.; Freitas, R.C.; Engler, M.L.P.D.; Edelhoff, D.; Klaus, G.; Prandtner, O.; Berthold, M.; Liebermann, A. Historical development of root analogue implants: A review of published papers. Br. J. Oral Maxillofac. Surg. 2019, 57, 496–504.
- Wojda, S.M. Comparative Analysis of Two Methods of Assessment Wear of Dental Materials. Acta Mech. Autom. 2015, 9, 105–109.
- Duhn, C.; Thalji, G.; Al-Tarwaneh, S.; Cooper, L.F. A digital approach to robust and esthetic implant overdenture construction. J. Esthet. Restor. Dent. 2021, 33, 118–126.
- Deb, S.; Chana, S. Biomaterials in Relation to Dentistry. Front. Oral Biol. 2015, 17, 1–12.
- Orsini, G.; Pagella, P.; Mitsiadis, T.A. Modern Trends in Dental Medicine: An Update for Internists. Am. J. Med. 2018, 131, 1425–1430.
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2000 2019, 79, 178–189.
- Zhang, Y.; Kelly, J.R. Dental Ceramics for Restoration and Metal Veneering. Dent. Clin. N. Am. 2017, 61, 797–819.
- Bhatavadekar, N.B.; Gharpure, A.S.; Balasubramanium, N.; Scheyer, E.T. In Vitro Surface Testing Methods for Dental Implants-Interpretation and Clinical Relevance: A Review. Compend. Contin. Educ. Dent. 2020, 41, e1–e9.
- Meschi, N.; Patel, B.; Ruparel, N.B. Material Pulp Cells and Tissue Interactions. J. Endod. 2020, 46, S150–S160.
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258.
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D printing restorative materials using a stereolithographic technique: A systematic review. Dent. Mater. 2021, 37, 336–350.
- Wang, Y.; Bäumer, D.; Ozga, A.K.; Körner, G.; Bäumer, A. Patient satisfaction and oral health-related quality of life 10 years after implant placement. BMC Oral Health 2021, 21, 30.
- Eick, S. Biofilms. Monogr. Oral Sci. 2020, 29, 1–11.
- Díaz-Garrido, N.; Lozano, C.P.; Kreth, J.; Giacaman, R.A. Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis. Appl. Environ. Microbiol. 2020, 86.
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260.
- Bhadila, G.; Menon, D.; Wang, X.; Vila, T.; Melo, M.A.S.; Montaner, S.; Arola, D.D.; Weir, M.D.; Sun, J.; Xu, H.H.K. Long-term antibacterial activity and cytocompatibility of novel low-shrinkage-stress, remineralizing composites. J. Biomater. Sci. Polym. Ed. 2021, 1–16.
- Tettamanti, L.; Cura, F.; Andrisani, C.; Bassi, M.A.; Silvestre-Rangil, J.; Tagliabue, A. A new implant-abutment connection for bacterial microleakage prevention: An in vitro study. ORAL Implantol. 2017, 10, 172–180.
- Revilla-León, M.; Morillo, J.A.; Att, W.; Özcan, M. Chemical Composition, Knoop Hardness, Surface Roughness, and Adhesion Aspects of Additively Manufactured Dental Interim Materials. J. Prosthodont. 2020.
- Ercoli, C.; Caton, J.G. Dental prostheses and tooth-related factors. J. Periodontol. 2018, 89, S223–S236.
- Revilla-León, M.; Husain, N.A.H.; Methani, M.M.; Özcan, M. Chemical composition, surface roughness, and ceramic bond strength of additively manufactured cobalt-chromium dental alloys. J. Prosthet. Dent. 2020.
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57.
- Preoteasa, E.A.; Preoteasa, E.S.; Suciu, I.; Bartok, R.N. Atomic and nuclear surface analysis methods for dental materials: A review. AIMS Mater. Sci. 2018, 5, 781–844.
- Karova, E. Application of Atomic Force Microscopy in Dental Investigations. Int. J. Sci. Res. 2020, 9, 1319–1326.
- Roa, J.J.; Oncins, G.; Díaz, J.; Capdevila, X.G.; Sanz, F.; Segarra, M. Study of the friction, adhesion and mechanical properties of single crystals, ceramics and ceramic coatings by AFM. J. Eur. Ceram. Soc. 2011, 31, 429–449.
- Vilá, J.F.; García, J.C.; Guestrin, A. SEM Applied to the Development of Bioactive Surface of Dental Implants. Microsc. Microanal. 2020, 26, 147–148.
- Banaszek, K.; Sawicki, J.; Wołowiec-Korecka, E.; Gorzędowski, J.; Danowska-Klonowska, D.; Sokołowski, J. Use of optical microscopy for evaluation of tooth structure. J. Achiev. Mater. Manuf. Eng. 2016, 79, 31–40.
- Chander, N.G. Characterization of dental materials. J. Indian Prosthodont. Soc. 2018, 18, 289–290.
- Zhou, X.; Thompson, G.E. Electron and Photon Based Spatially Resolved Techniques. In Reference Module in Materials Science and Materials Engineering; Elsevier: New York, NY, USA, 2017; pp. 1–30.
- Yanikoglu, N.D.; Sakarya, R.E. Test methods used in the evaluation of the structure features of the restorative materials: A literature review. J. Mater. Res. Technol. 2020, 9, 9720–9734.
- Naves, L.Z.; Gerdolle, D.A.; de Andrade, O.S.; Markus Maria Gresnigt, M. Seeing is believing? When scanning electron microscopy (SEM) meets clinical dentistry: The replica technique. Microsc. Res. Tech. 2020, 83, 1118–1123.
- Siekaniec, D.; Kopyciński, D. Analysis Phases and Crystallographic Orientation of the grain of High Chromium Cast Iron Using EBSD Technique. J. Cast. Mater. Eng. 2017, 1, 15.
- Koblischka-Veneva, A.; Koblischka, M.R.; Schmauch, J.; Hannig, M. Comparison of human and bovine dental enamel by TEM and t-EBSD investigations. IOP Conf. Ser. Mater. Sci. Eng. 2019, 625, 012006.
- Tomota, Y. Crystallographic characterization of steel microstructure using neutron diffraction. Sci. Technol. Adv. Mater. 2019, 20, 1189–1206.
- Shukla, A.K. Electron Spin Resonance Spectroscopy in Medicine; Shukla, A.K., Ed.; Springer: Singapore, 2018; ISBN 9789811322303.
- Pandoleon, P.; Kontonasaki, E.; Kantiranis, N.; Pliatsikas, N.; Patsalas, P.; Papadopoulou, L.; Zorba, T.; Paraskevopoulos, K.M.; Koidis, P. Aging of 3Y-TZP dental zirconia and yttrium depletion. Dent. Mater. 2017, 33, e385–e392.
- Lopes, C.d.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769.
- Lach, S.; Jurczak, P.; Karska, N.; Kubiś, A.; Szymańska, A.; Rodziewicz-Motowidło, S. Spectroscopic methods used in implant material studies. Molecules 2020, 25, 579.
- Kreve, S.; Cândido dos Reis, A. Influence of the electrostatic condition of the titanium surface on bacterial adhesion: A systematic review. J. Prosthet. Dent. 2021, 125, 416–420.
- Kim, I.H.; Son, J.S.; Min, B.K.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. A simple, sensitive and non-destructive technique for characterizing bovine dental enamel erosion: Attenuated total reflection Fourier transform infrared spectroscopy. Int. J. Oral Sci. 2016, 8, 54–60.
- Furuhashi, K.; Uo, M.; Kitagawa, Y.; Watari, F. Rapid and non-destructive analysis of metallic dental restorations using X-ray fluorescence spectra and light-element sampling tools. Appl. Surf. Sci. 2012, 262, 13–18.
- Malik, A.K.; Kumar, R. Heena Spectroscopy: Types. In Encyclopedia of Food and Health; Elsevier Ltd.: New York, NY, USA, 2015; pp. 64–72. ISBN 9780123849533.
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of peptide/protein self-assembly and aggregation by spectroscopic methods. Molecules 2020, 25, 4854.
- Kafle, B.P. Infrared (IR) spectroscopy. In Chemical Analysis and Material Characterization by Spectrophotometry; Elsevier: New York, NY, USA, 2020; pp. 199–243.
- Smith, E.; Dent, G. Modern Raman Spectroscopy—A Practical Approach. John Wiley and Sons Ltd.: Chichester, UK, 2005; p. 210. ISBN 0471496685.
- Dolenko, G.N.; Poleshchuk, O.K.; Latosińska, J.N. X-ray emission spectroscopy, methods. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: New York, NY, USA, 2016; pp. 691–694. ISBN 9780128032244.
- Sharma, R.K. Various Spectroscopic Techniques. In Environmental Pollution: Monitoring Modelling and Control; Studium Press, LLC: Houston, TX, USA, 2017; pp. 181–206. ISBN ISBN:1626991022/978-1626991026.
- Patonay, G.; Beckford, G.; Hänninen, P. UV-Vis and NIR Fluorescence Spectroscopy. In Handbook of Spectroscopy, 2nd Enlarged ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; Volume 3–4, pp. 999–1036. ISBN 9783527654703.
- Adams, F.C. X-ray absorption and Diffraction | Overview. In Encyclopedia of Analytical Science; Elsevier: New York, NY, USA, 2019; pp. 391–403. ISBN 9780081019832.
- Brader, M.L. UV-absorbance, fluorescence and FT-IR spectroscopy in biopharmaceutical development. In Biophysical Characterization of Proteins in Developing Biopharmaceuticals; Elsevier: New York, NY, USA, 2020; pp. 97–121. ISBN 9780444641731.
- Akhtar, S.; Ali, S. Characterization of nanomaterials: Techniques and tools. In Applications of Nanomaterials in Human Health; Springer: Singapore, 2020; pp. 23–43. ISBN 9789811548024.
- Fa, K.; Jiang, T.; Nalaskowski, J.; Miller, J.D. Optical and spectroscopic characteristics of oleate adsorption as revealed by FTIR analysis. Langmuir 2004, 20, 5311–5321.
- Le Pevelen, D.D.; Tranter, G.E. FT-IR and raman spectroscopies, polymorphism applications. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: New York, NY, USA, 2016; pp. 750–761. ISBN 9780128032244.
- Bell, S.E.J.; Xu, Y. Infrared spectroscopy|Industrial applications. In Encyclopedia of Analytical Science; Elsevier: New York, NY, USA, 2019; pp. 124–133. ISBN 9780081019832.
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972.
- Puspita, S.; Sunarintyas, S.; Mulyawati, E.; Anwar, C.; Sukirno; Soesatyo, M.H.N.E. Molecular weight determination and structure identification of Bombyx mori L. Fibroin as material in dentistry. In AIP Conference Proceedings; American Institute of Physics Inc.: College, MA, USA, 2020; Volume 2260, p. 40018.
- Rafeek, A.D.; Choi, G.; Evans, L.A. Morphological, spectroscopic and crystallographic studies of calcium phosphate bioceramic powders. J. Aust. Ceram. Soc. 2018, 54, 161–168.
- Dutta, A. Fourier Transform Infrared Spectroscopy. In Spectroscopic Methods for Nanomaterials Characterization; Elsevier: New York, NY, USA, 2017; Volume 2, pp. 73–93. ISBN 9780323461467.
- Rosi, F.; Cartechini, L.; Sali, D.; Miliani, C. Recent trends in the application of fourier transform infrared (FT-IR) spectroscopy in Heritage Science: From micro: From non-invasive FT-IR. Phys. Sci. Rev. 2019, 4.
- Margariti, C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralised excavated textiles. Herit. Sci. 2019, 7, 1–14.
- Munajad, A.; Subroto, C. Suwarno Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies 2018, 11, 364.
- Puppin-Rontani, J.; Fugolin, A.P.P.; Costa, A.R.; Correr-Sobrinho, L.; Pfeifer, C.S. In vitro performance of 2-step, total etch adhesives modified by thiourethane additives. Int. J. Adhes. Adhes. 2020, 103, 102688.
- Shim, J.S.; Lee, S.Y.; Song, S.-Y.; Jha, N.; Ryu, J.J. Polymerization efficiency of dental dual-cured resin cement light-cured at various times after the initiation of chemical activation. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 622–628.
- Fugolin, A.P.; Lewis, S.; Logan, M.G.; Ferracane, J.L.; Pfeifer, C.S. Methacrylamide–methacrylate hybrid monomers for dental applications. Dent. Mater. 2020, 36, 1028–1037.
- Fugolin, A.P.; Dobson, A.; Ferracane, J.L.; Pfeifer, C.S. Effect of residual solvent on performance of acrylamide-containing dental materials. Dent. Mater. 2019, 35, 1378–1387.
- Fugolin, A.P.P.; Navarro, O.; Logan, M.G.; Huynh, V.; França, C.M.; Ferracane, J.L.; Pfeifer, C.S. Synthesis of di- and triacrylamides with tertiary amine cores and their evaluation as monomers in dental adhesive interfaces. Acta Biomater. 2020, 115, 148–159.
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, ester-free monomers: Polymerization kinetics, mechanical properties, biocompatibility and anti-biofilm activity. Acta Biomater. 2019, 100, 132–141.
- Alania, Y.; dos Reis, M.C.; Nam, J.-W.; Phansalkar, R.S.; McAlpine, J.; Chen, S.-N.; Pauli, G.F.; Bedran-Russo, A.K. A dynamic mechanical method to assess bulk viscoelastic behavior of the dentin extracellular matrix. Dent. Mater. 2020, 36, 1536–1543.
- Zhang, P.; Zhao, X.M. Synthesis, crystal structure and bioactivity evaluation of a heterocyclic compound. Jiegou Huaxue 2020, 39, 1892–1897.
- Seredin, P.V.; Uspenskaya, O.A.; Goloshchapov, D.L.; Ippolitov, I.Y.; Vongsvivut, J.; Ippolitov, Y.A. Organic-mineral interaction between biomimetic materials and hard dental tissues. Sovrem. Tehnol. V Med. 2020, 12, 43–51.
- Gurgenc, T. Structural characterization and dielectrical properties of Ag-doped nano-strontium apatite particles produced by hydrothermal method. J. Mol. Struct. 2021, 1223, 128990.
- Jing, X.; Xie, B.; Li, X.; Dai, Y.; Nie, L.; Li, C. Peptide decorated demineralized dentin matrix with enhanced bioactivity, osteogenic differentiation via carboxymethyl chitosan. Dent. Mater. 2021, 37, 19–29.
- Ramos, N.C.; Alves, L.M.M.; Ricco, P.; Santos, G.M.A.S.; Bottino, M.A.; Campos, T.M.B.; Melo, R.M. Strength and bondability of a dental Y-TZP after silica sol-gel infiltrations. Ceram. Int. 2020, 46, 17018–17024.
- Asadi, F.; Forootanfar, H.; Ranjbar, M. A facile one-step preparation of Ca10(PO4)6(OH)2/Li-BioMOFs resin nanocomposites with Glycyrrhiza glabra (licorice) root juice as green capping agent and mechanical properties study. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1331–1339.
- Fu, D.; Lu, Y.; Gao, S.; Peng, Y.; Duan, H. Chemical Property and Antibacterial Activity of Metronidazole-decorated Ti through Adhesive Dopamine. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 968–972.
- Yakufu, M.; Wang, Z.; Wang, Y.; Jiao, Z.; Guo, M.; Liu, J.; Zhang, P. Covalently functionalized poly(etheretherketone) implants with osteogenic growth peptide (OGP) to improve osteogenesis activity. RSC Adv. 2020, 10, 9777–9785.
- Ding, Y.; Zhang, H.; Wang, X.; Zu, H.; Wang, C.; Dong, D.; Lyu, M.; Wang, S. Immobilization of Dextranase on Nano-Hydroxyapatite as a Recyclable Catalyst. Materials 2020, 14, 130.
- Roopavath, U.K.; Sah, M.K.; Panigrahi, B.B.; Rath, S.N. Mechanochemically synthesized phase stable and biocompatible β-tricalcium phosphate from avian eggshell for the development of tissue ingrowth system. Ceram. Int. 2019, 45, 12910–12919.
- Zeng, W.; Liu, F.; He, J. Physicochemical Properties of Bis-GMA/TEGDMA Dental Resin Reinforced with Silanized Multi-Walled Carbon Nanotubes. Silicon 2019, 11, 1345–1353.
- Voicu, G.; Didilescu, A.C.; Stoian, A.B.; Dumitriu, C.; Greabu, M.; Andrei, M. Mineralogical and microstructural characteristics of two dental pulp capping materials. Materials 2019, 12, 1772.
- Yushau, U.S.; Almofeez, L.; Bozkurt, A. Novel Polymer Nanocomposites Comprising Triazole Functional Silica for Dental Application. Silicon 2020, 12, 109–116.
- Agha, A.; Parker, S.; Patel, M. Polymerization shrinkage kinetics and degree of conversion of commercial and experimental resin modified glass ionomer luting cements (RMGICs). Dent. Mater. 2020, 36, 893–904.
- Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A.; Trejo-Carbajal, N.; Herrera-González, A.M. Evaluation of new coinitiators of camphorquinone useful in the radical photopolymerization of dental monomers. J. Photochem. Photobiol. A Chem. 2020, 403, 112844.
- Alotaibi, J.; Saji, S.; Swain, M.V. FTIR characterization of the setting reaction of biodentineTM. Dent. Mater. 2018, 34, 1645–1651.
- Dinesh Kumar, S.; Mohamed Abudhahir, K.; Selvamurugan, N.; Vimalraj, S.; Murugesan, R.; Srinivasan, N.; Moorthi, A. Formulation and biological actions of nano-bioglass ceramic particles doped with Calcarea phosphorica for bone tissue engineering. Mater. Sci. Eng. C 2018, 83, 202–209.
- Alqahtani, M. Effect of hexagonal boron nitride nanopowder reinforcement and mixing methods on physical and mechanical properties of self-cured PMMA for dental applications. Materials 2020, 13, 2323.
This entry is offline, you can click here to edit this entry!
