Malignant pleural mesothelioma (MPM) is a rare and highly aggressive disease that arises from pleural mesothelial cells, characterized by a median survival of approximately 13–15 months after diagnosis. The primary cause of this disease is asbestos exposure and the main issues associated with it are late diagnosis and lack of effective therapies. Asbestos-induced cellular damage is associated with the generation of an inflammatory microenvironment that influences and supports tumor growth, possibly in association with patients’ genetic predisposition and tumor genomic profile. The chronic inflammatory response to asbestos fibers leads to a unique tumor immune microenvironment (TIME) composed of a heterogeneous mixture of stromal, endothelial, and immune cells, and relative composition and interaction among them is suggested to bear prognostic and therapeutic implications. TIME in MPM is known to be constituted by immunosuppressive cells, such as type 2 tumor-associated macrophages and T regulatory lymphocytes, plus the expression of several immunosuppressive factors, such as tumor-associated PD-L1. Several studies in recent years have contributed to achieve a greater understanding of the pathogenetic mechanisms in tumor development and pathobiology of TIME, that opens the way to new therapeutic strategies. The study of TIME is fundamental in identifying appropriate prognostic and predictive tissue biomarkers. In the present review, we summarize the current knowledge about the pathological characterization of TIME in MPM.
- mesothelioma
- tumor microenvironment
- tumor-associated macrophages
- dendritic cells
- immunohistochemistry
1. Introduction
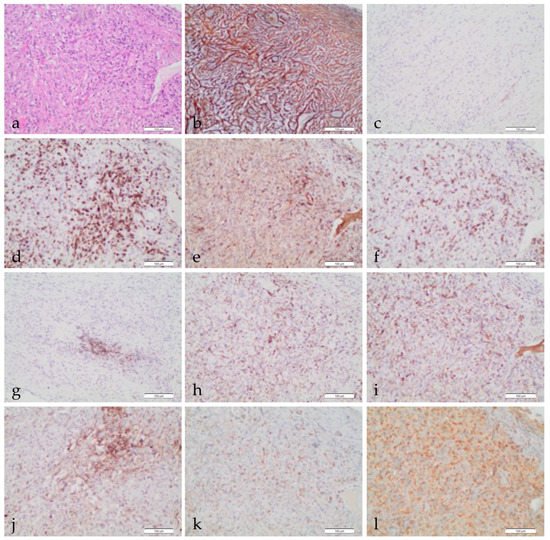
2. The Tumor Immune Microenvironment
3. Angiogenesis
4. Conclusions
TIME is a challenging component with an emerging pathogenic, immunomodulatory, and growth-promoting role in MPM. Given the relatively low mutational burden of MPM, biological events other than genetics may be critical determinants of MPM growth and aggressiveness and could influence cells’ immune-escape.
A greater understanding of infiltrating immune cells, their role and function, and the presence of ligand or modulatory marker expression will give a wider and better structured picture of the tumor–immune cell interplayReferences
- Yang, ; Testa, J.R.; Carbone, M. Mesothelioma Epidemiology, Carcinogenesis, and Pathogenesis. Curr. Treat. Opt. Oncol. 2008, 9, 147–157, doi:10.1007/s11864-008-0067-z.
- Kindler, L.; Ismaila, N.; Armato, S.G.; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. JCO 2018, 36, 1343–1373, doi:10.1200/JCO.2017.76.6394.
- Nicholson, G.; Sauter, J.L.; Nowak, A.K.; Kindler, H.L.; Gill, R.R.; Remy-Jardin, M.; Armato, S.G.; Fernandez-Cuesta, L.; Bueno, R.; Alcala, N.; et al. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J. Thorac. Oncol. 2020, 15, 29–49, doi:10.1016/j.jtho.2019.08.2506.
- Opitz, ; Scherpereel, A.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO Guidelines for the Management of Malignant Pleural Mesothelioma. Eur. J. Cardio Thorac. Surg. 2020, 58, 1–24, doi:10.1093/ejcts/ezaa158.
- Van Gerwen, ; Alpert, N.; Wolf, A.; Ohri, N.; Lewis, E.; Rosenzweig, K.E.; Flores, R.; Taioli, E. Prognostic Factors of Survival in Patients with Malignant Pleural Mesothelioma: An Analysis of the National Cancer Database. Carcinogenesis 2019, 40, 529–536, doi:10.1093/carcin/bgz004.
- Vogelzang, J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644, doi:10.1200/JCO.2003.11.136.
- Baas, ; Fennell, D.; Kerr, K.M.; Van Schil, P.E.; Haas, R.L.; Peters, S. ESMO Guidelines Committee Malignant Pleural Mesothelioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26 (Suppl. S5), v31–v39, doi:10.1093/annonc/mdv199.
- Bueno, ; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive Genomic Analysis of Malignant Pleural Mesothelioma Identifies Recurrent Mutations, Gene Fusions and Splicing Alterations. Nat. Genet. 2016, 48, 407–416, doi:10.1038/ng.3520.
- Cakiroglu, ; Senturk, S. Genomics and Functional Genomics of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2020, 21, 6342, doi:10.3390/ijms21176342.
- Guo, ; Chmielecki, J.; Goparaju, C.; Heguy, A.; Dolgalev, I.; Carbone, M.; Seepo, S.; Meyerson, M.; Pass, H.I. Whole-Exome Sequencing Reveals Frequent Genetic Alterations in BAP1, NF2, CDKN2A, and CUL1 in Malignant Pleural Mesothelioma. Cancer Res. 2015, 75, 264–269, doi:10.1158/0008-5472.CAN-14-1008.
- Hmeljak, ; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565, doi:10.1158/2159-8290.CD-18-0804.
- Kang, C.; Kim, H.K.; Lee, S.; Mendez, P.; Kim, J.W.; Woodard, G.; Yoon, J.-H.; Jen, K.-Y.; Fang, L.T.; Jones, K.; et al. Whole Exome and Targeted Deep Sequencing Identify Genome-Wide Allelic Loss and Frequent SETDB1 Mutations in Malignant Pleural Mesotheliomas. Oncotarget 2016, 7, 8321–8331, doi:10.18632/oncotarget.7032.
- Lo Iacono, ; Monica, V.; Righi, L.; Grosso, F.; Libener, R.; Vatrano, S.; Bironzo, P.; Novello, S.; Musmeci, L.; Volante, M.; et al. Targeted Next-Generation Sequencing of Cancer Genes in Advanced Stage Malignant Pleural Mesothelioma: A Retrospective Study. J. Thorac. Oncol. 2015, 10, 492–499, doi:10.1097/JTO.0000000000000436.
- Bott, ; Brevet, M.; Taylor, B.S.; Shimizu, S.; Ito, T.; Wang, L.; Creaney, J.; Lake, R.A.; Zakowski, M.F.; Reva, B.; et al. The Nuclear Deubiquitinase BAP1 Is Commonly Inactivated by Somatic Mutations and 3p21.1 Losses in Malignant Pleural Mesothelioma. Nat. Genet. 2011, 43, 668–672, doi:10.1038/ng.855.
- Testa, R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 Mutations Predispose to Malignant Mesothelioma. Nat. Genet. 2011, 43, 1022–1025, doi:10.1038/ng.912.
- Matsuzaki, ; Maeda, M.; Lee, S.; Nishimura, Y.; Kumagai-Takei, N.; Hayashi, H.; Yamamoto, S.; Hatayama, T.; Kojima, Y.; Tabata, R.; et al. Asbestos-Induced Cellular and Molecular Alteration of Immunocompetent Cells and Their Relationship with Chronic Inflammation and Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 492608, doi:10.1155/2012/492608.
- Kusamura, ; Kepenekian, V.; Villeneuve, L.; Lurvink, R.J.; Govaerts, K.; De Hingh, I.H.J.T.; Moran, B.J.; Van der Speeten, K.; Deraco, M.; Glehen, O.; et al. Peritoneal Mesothelioma: PSOGI/EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Eur. J. Surg. Oncol. 2021, 47, 36–59, doi:10.1016/j.ejso.2020.02.011.
- Benzerdjeb, ; Dartigues, P.; Kepenekian, V.; Valmary-Degano, S.; Mery, E.; Avérous, G.; Chevallier, A.; Laverriere, M.-H.; Villa, I.; Harou, O.; et al. Tertiary Lymphoid Structures in Epithelioid Malignant Peritoneal Mesothelioma Are Associated with Neoadjuvant Chemotherapy, but Not with Prognosis. Virchows Arch. 2021, doi:10.1007/s00428-021-03099-1.
- Baas, ; Scherpereel, A.; Nowak, A.; Fujimoto, N.; Peters, S.; Tsao, A.; Mansfield, A.; Popat, S.; Jahan, T.; Antonia, S.; et al. ID:2908 First-Line Nivolumab + Ipilimumab vs. Chemotherapy in Unresectable Malignant Pleural Mesothelioma: CheckMate 743. J. Thorac. Oncol. 2020, 15, e42, doi:10.1016/j.jtho.2020.08.004.
- Fennell, ; Ottensmeier, C.; Califano, R.; Hanna, G.; Ewings, S.; Hill, K.; Wilding, S.; Danson, S.; Nye, M.; Steele, N.; et al. PS01.11 Nivolumab Versus Placebo in Relapsed Malignant Mesothelioma: The CONFIRM Phase 3 Trial. J. Thorac. Oncol. 2021, 16, S62, doi:10.1016/j.jtho.2021.01.323.
- Castelletti, ; Yeo, D.; van Zandwijk, N.; Rasko, J.E.J. Anti-Mesothelin CAR T Cell Therapy for Malignant Mesothelioma. Biomark. Res. 2021, 9, 11, doi:10.1186/s40364-021-00264-1.
- Hiltbrunner, ; Britschgi, C.; Schuberth, P.; Bankel, L.; Nguyen-Kim, T.D.L.; Gulati, P.; Weder, W.; Opitz, I.; Lauk, O.; Caviezel, C.; et al. Local Delivery of CAR T Cells Targeting Fibroblast Activation Protein Is Safe in Patients with Pleural Mesothelioma: First Report of FAPME, a Phase I Clinical Trial. Ann. Oncol. 2021, 32, 120–121, doi:10.1016/j.annonc.2020.10.474.
- Lau, P.; van Montfoort, N.; Kinderman, P.; Lukkes, M.; Klaase, L.; van Nimwegen, M.; van Gulijk, M.; Dumas, J.; Mustafa, D.A.M.; Lievense, S.L.A.; et al. Dendritic Cell Vaccination and CD40-Agonist Combination Therapy Licenses T Cell-Dependent Antitumor Immunity in a Pancreatic Carcinoma Murine Model. J. Immunother. Cancer 2020, 8, e000772, doi:10.1136/jitc-2020-000772.
- Parra, R.; Zhai, J.; Tamegnon, A.; Zhou, N.; Pandurengan, R.K.; Barreto, C.; Jiang, M.; Rice, D.C.; Creasy, C.; Vaporciyan, A.A.; et al. Identification of Distinct Immune Landscapes Using an Automated Nine-Color Multiplex Immunofluorescence Staining Panel and Image Analysis in Paraffin Tumor Tissues. Sci. Rep. 2021, 11, 4530, doi:10.1038/s41598-021-83858-x.
- Ijsselsteijn, E.; van der Breggen, R.; Farina Sarasqueta, A.; Koning, F.; de Miranda, N.F.C.C. A 40-Marker Panel for High Dimensional Characterization of Cancer Immune Microenvironments by Imaging Mass Cytometry. Front. Immunol. 2019, 10, 2534, doi:10.3389/fimmu.2019.02534.
- Mungenast, ; Fernando, A.; Nica, R.; Boghiu, B.; Lungu, B.; Batra, J.; Ecker, R.C. Next-Generation Digital Histopathology of the Tumor Microenvironment. Genes 2021, 12, 538, doi:10.3390/genes12040538.
- De Perrot, ; Wu, L.; Cabanero, M.; Perentes, J.Y.; McKee, T.D.; Donahoe, L.; Bradbury, P.; Kohno, M.; Chan, M.-L.; Murakami, J.; et al. Prognostic Influence of Tumor Microenvironment after Hypofractionated Radiation and Surgery for Mesothelioma. J. Thorac. Cardiovasc. Surg. 2020, 159, 2082–2091.e1, doi:10.1016/j.jtcvs.2019.10.122.
- Suzuki, ; Kadota, K.; Sima, C.S.; Sadelain, M.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. Chronic Inflammation in Tumor Stroma Is an Independent Predictor of Prolonged Survival in Epithelioid Malignant Pleural Mesothelioma Patients. Cancer Immunol. Immunother. 2011, 60, 1721–1728, doi:10.1007/s00262-011-1073-8.
- Ujiie, ; Kadota, K.; Nitadori, J.-I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The Tumoral and Stromal Immune Microenvironment in Malignant Pleural Mesothelioma: A Comprehensive Analysis Reveals Prognostic Immune Markers. Oncoimmunology 2015, 4, e1009285, doi:10.1080/2162402X.2015.1009285.
- Lievense, A.; Bezemer, K.; Cornelissen, R.; Kaijen-Lambers, M.E.H.; Hegmans, J.P.J.J.; Aerts, J.G.J.V. Precision Immunotherapy; Dynamics in the Cellular Profile of Pleural Effusions in Malignant Mesothelioma Patients. Lung Cancer 2017, 107, 36–40, doi:10.1016/j.lungcan.2016.04.015.
- Yoshihara, ; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612, doi:10.1038/ncomms3612.
- Xu, ; Cheng, L.; Fan, Y.; Mao, W. Tumor Microenvironment-Associated Immune-Related Genes for the Prognosis of Malignant Pleural Mesothelioma. Front. Oncol. 2020, 10, 544789, doi:10.3389/fonc.2020.544789.
- Lee, -S.; Jang, H.-J.; Choi, J.M.; Zhang, J.; de Rosen, V.L.; Wheeler, T.M.; Lee, J.-S.; Tu, T.; Jindra, P.T.; Kerman, R.H.; et al. Comprehensive Immunoproteogenomic Analyses of Malignant Pleural Mesothelioma. JCI Insight 2018, 3, doi:10.1172/jci.insight.98575.
- Gordon, J.; Rockwell, G.N.; Jensen, R.V.; Rheinwald, J.G.; Glickman, J.N.; Aronson, J.P.; Pottorf, B.J.; Nitz, M.D.; Richards, W.G.; Sugarbaker, D.J.; et al. Identification of Novel Candidate Oncogenes and Tumor Suppressors in Malignant Pleural Mesothelioma Using Large-Scale Transcriptional Profiling. Am. J. Pathol. 2005, 166, 1827–1840, doi:10.1016/S0002-9440(10)62492-3.
- Blum, ; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting Heterogeneity in Malignant Pleural Mesothelioma through Histo-Molecular Gradients for Clinical Applications. Nat. Commun. 2019, 10, 1333, doi:10.1038/s41467-019-09307-6.
- Patil, S.; Righi, L.; Koeppen, H.; Zou, W.; Izzo, S.; Grosso, F.; Libener, R.; Loiacono, M.; Monica, V.; Buttigliero, C.; et al. Molecular and Histopathological Characterization of the Tumor Immune Microenvironment in Advanced Stage of Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 124–133, doi:10.1016/j.jtho.2017.09.1968.
- Liu, ; Qian, K.; Lu, G.; Chen, P.; Zhang, Y. Identification of Genes and Pathways Involved in Malignant Pleural Mesothelioma Using Bioinformatics Methods. BMC Med. Genom. 2021, 14, 104, doi:10.1186/s12920-021-00954-7.
- Duan, ; Wang, K.; Duan, Y.; Chen, X.; Chu, X.; Hu, P.; Xiong, B. Combined Analysis of RNA Sequence and Microarray Data Reveals a Competing Endogenous RNA Network as Novel Prognostic Markers in Malignant Pleural Mesothelioma. Front. Oncol. 2021, 11, 615234, doi:10.3389/fonc.2021.615234.
- Morani, ; Bisceglia, L.; Rosini, G.; Mutti, L.; Melaiu, O.; Landi, S.; Gemignani, F. Identification of Overexpressed Genes in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 2738, doi:10.3390/ijms22052738.
- Liu, ; Klominek, J. Regulation of Matrix Metalloprotease Activity in Malignant Mesothelioma Cell Lines by Growth Factors. Thorax 2003, 58, 198–203, doi:10.1136/thorax.58.3.198.
- Scarpa, ; Giuffrida, A.; Fazi, M.; Coletti, A.; Palumbo, C.; Pass, H.I.; Procopio, A.; Modesti, A. Migration of Mesothelioma Cells Correlates with Histotype-Specific Synthesis of Extracellular Matrix. Int. J. Mol. Med. 1999, 4, 67–71, doi:10.3892/ijmm.4.1.67.
- Jagirdar, M.; Papazoglou, E.D.; Pitaraki, E.; Kouliou, O.A.; Rouka, E.; Giannakou, L.; Giannopoulos, S.; Sinis, S.I.; Hatzoglou, C.; Gourgoulianis, K.I.; et al. Cell and Extracellular Matrix Interaction Models in Benign Mesothelial and Malignant Pleural Mesothelioma Cells in 2D and 3D In-Vitro. Clin. Exp. Pharmacol. Physiol. 2021, 48, 543–552, doi:10.1111/1440-1681.13446.
- Abayasiriwardana, S.; Wood, M.K.; Prêle, C.M.; Birnie, K.A.; Robinson, B.W.; Laurent, G.J.; McAnulty, R.J.; Mutsaers, S.E. Inhibition of Collagen Production Delays Malignant Mesothelioma Tumor Growth in a Murine Model. Biochem. Biophys. Res. Commun. 2019, 510, 198–204, doi:10.1016/j.bbrc.2019.01.057.
- Balancin, L.; Teodoro, W.R.; Farhat, C.; de Miranda, T.J.; Assato, A.K.; de Souza Silva, N.A.; Velosa, A.P.; Falzoni, R.; Ab’Saber, A.M.; Roden, A.C.; et al. An Integrative Histopathologic Clustering Model Based on Immuno-Matrix Elements to Predict the Risk of Death in Malignant Mesothelioma. Cancer Med. 2020, 9, 4836–4849, doi:10.1002/cam4.3111.
- Balancin, L.; Teodoro, W.R.; Baldavira, C.M.; Prieto, T.G.; Farhat, C.; Velosa, A.P.; da Costa Souza, P.; Yaegashi, L.B.; Ab’Saber, A.M.; Takagaki, T.Y.; et al. Different Histological Patterns of Type-V Collagen Levels Confer a Matrices-Privileged Tissue Microenvironment for Invasion in Malignant Tumors with Prognostic Value. Pathol. Res. Pract. 2020, 216, 153277, doi:10.1016/j.prp.2020.153277.
- Galateau Salle, ; Le Stang, N.; Nicholson, A.G.; Pissaloux, D.; Churg, A.; Klebe, S.; Roggli, V.L.; Tazelaar, H.D.; Vignaud, J.M.; Attanoos, R.; et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J. Thorac. Oncol. 2018, 13, 1189–1203, doi:10.1016/j.jtho.2018.04.023.
- Galateau Salle, ; Le Stang, N.; Tirode, F.; Courtiol, P.; Nicholson, A.G.; Tsao, M.-S.; Tazelaar, H.D.; Churg, A.; Dacic, S.; Roggli, V.; et al. Comprehensive Molecular and Pathologic Evaluation of Transitional Mesothelioma Assisted by Deep Learning Approach: A Multi-Institutional Study of the International Mesothelioma Panel from the MESOPATH Reference Center. J. Thorac. Oncol. 2020, 15, 1037–1053, doi:10.1016/j.jtho.2020.01.025.
- LeBleu, S.; Kalluri, R. A Peek into Cancer-Associated Fibroblasts: Origins, Functions and Translational Impact. Dis. Models Mech. 2018, 11, dmm029447, doi:10.1242/dmm.029447.
- Ohara, ; Enomoto, A.; Tsuyuki, Y.; Sato, K.; Iida, T.; Kobayashi, H.; Mizutani, Y.; Miyai, Y.; Hara, A.; Mii, S.; et al. Connective Tissue Growth Factor Produced by Cancer-Associated Fibroblasts Correlates with Poor Prognosis in Epithelioid Malignant Pleural Mesothelioma. Oncol. Rep. 2020, 44, 838–848, doi:10.3892/or.2020.7669.
- Katoh, ; Nakagama, H. FGF Receptors: Cancer Biology and Therapeutics. Med. Res. Rev. 2014, 34, 280–300, doi:10.1002/med.21288.
- Marek, A.; Hinz, T.K.; von Mässenhausen, A.; Olszewski, K.A.; Kleczko, E.K.; Boehm, D.; Weiser-Evans, M.C.; Nemenoff, R.A.; Hoffmann, H.; Warth, A.; et al. Nonamplified FGFR1 Is a Growth Driver in Malignant Pleural Mesothelioma. Mol. Cancer Res. 2014, 12, 1460–1469, doi:10.1158/1541-7786.MCR-14-0038.
- Blackwell, ; Sherk, C.; Fricko, M.; Ganji, G.; Barnette, M.; Hoang, B.; Tunstead, J.; Skedzielewski, T.; Alsaid, H.; Jucker, B.M.; et al. Inhibition of FGF/FGFR Autocrine Signaling in Mesothelioma with the FGF Ligand Trap, FP-1039/GSK3052230. Oncotarget 2016, 7, 39861–39871, doi:10.18632/oncotarget.9515.
- Lam, -S.; Creaney, J.; Chen, F.K.; Chin, W.L.; Muruganandan, S.; Arunachalam, S.; Attia, M.S.; Read, C.; Murray, K.; Millward, M.; et al. A Phase II Trial of Single Oral FGF Inhibitor, AZD4547, as Second or Third Line Therapy in Malignant Pleural Mesothelioma. Lung Cancer 2020, 140, 87–92, doi:10.1016/j.lungcan.2019.12.018.
- Chen, ; Song, E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115, doi:10.1038/s41573-018-0004-1.
- Hegmans, P.J.J.; Hemmes, A.; Hammad, H.; Boon, L.; Hoogsteden, H.C.; Lambrecht, B.N. Mesothelioma Environment Comprises Cytokines and T-Regulatory Cells That Suppress Immune Responses. Eur. Respir. J. 2006, 27, 1086–1095, doi:10.1183/09031936.06.00135305.
- Schelch, ; Hoda, M.A.; Klikovits, T.; Münzker, J.; Ghanim, B.; Wagner, C.; Garay, T.; Laszlo, V.; Setinek, U.; Dome, B.; et al. Fibroblast Growth Factor Receptor Inhibition Is Active against Mesothelioma and Synergizes with Radio- and Chemotherapy. Am. J. Respir. Crit. Care Med. 2014, 190, 763–772, doi:10.1164/rccm.201404-0658OC.
- Kumar-Singh, ; Weyler, J.; Martin, M.J.; Vermeulen, P.B.; Van Marck, E. Angiogenic Cytokines in Mesothelioma: A Study of VEGF, FGF-1 and -2, and TGF Beta Expression. J. Pathol. 1999, 189, 72–78, doi:10.1002/(SICI)1096-9896(199909)189:1<72::AID-PATH401>3.0.CO;2-0.
- Righi, ; Cavallo, M.C.; Gatti, G.; Monica, V.; Rapa, I.; Busso, S.; Albera, C.; Volante, M.; Scagliotti, G.V.; Papotti, M. Tumor/Stromal Caveolin-1 Expression Patterns in Pleural Mesothelioma Define a Subgroup of the Epithelial Histotype with Poorer Prognosis. Am. J. Clin. Pathol. 2014, 141, 816–827, doi:10.1309/AJCP0F6WYBXGVDHX.
- Lolo, N.; Jiménez-Jiménez, V.; Sánchez-Álvarez, M.; Del Pozo, M.Á. Tumor-Stroma Biomechanical Crosstalk: A Perspective on the Role of Caveolin-1 in Tumor Progression. Cancer Metastasis Rev. 2020, 39, 485–503, doi:10.1007/s10555-020-09900-y.
- Albacete-Albacete, ; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM Deposition Is Driven by Caveolin-1-Dependent Regulation of Exosomal Biogenesis and Cargo Sorting. J. Cell Biol. 2020, 219, e202006178, doi:10.1083/jcb.202006178.
- Fujii, ; Toyoda, T.; Nakanishi, H.; Yatabe, Y.; Sato, A.; Matsudaira, Y.; Ito, H.; Murakami, H.; Kondo, Y.; Kondo, E.; et al. TGF-β Synergizes with Defects in the Hippo Pathway to Stimulate Human Malignant Mesothelioma Growth. J. Exp. Med. 2012, 209, 479–494, doi:10.1084/jem.20111653.
- Jiang, ; Yamashita, Y.; Chew, S.-H.; Akatsuka, S.; Ukai, S.; Wang, S.; Nagai, H.; Okazaki, Y.; Takahashi, T.; Toyokuni, S. Connective Tissue Growth Factor and β-Catenin Constitute an Autocrine Loop for Activation in Rat Sarcomatoid Mesothelioma. J. Pathol. 2014, 233, 402–414, doi:10.1002/path.4377.
- Ohara, ; Chew, S.H.; Misawa, N.; Wang, S.; Somiya, D.; Nakamura, K.; Kajiyama, H.; Kikkawa, F.; Tsuyuki, Y.; Jiang, L.; et al. Connective Tissue Growth Factor-Specific Monoclonal Antibody Inhibits Growth of Malignant Mesothelioma in an Orthotopic Mouse Model. Oncotarget 2018, 9, 18494–18509, doi:10.18632/oncotarget.24892.
- Richeldi, ; Fernández Pérez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an Anti-Connective Tissue Growth Factor Therapy, for Idiopathic Pulmonary Fibrosis (PRAISE): A Phase 2, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Respir. Med. 2020, 8, 25–33, doi:10.1016/S2213-2600(19)30262-0.
- Picozzi, J.; Pipas, J.M.; Koong, A.; Giaccia, A.; Bahary, N.; Krishnamurthi, S.S.; Lopez, C.D.; O’Dwyer, P.J.; Modelska, K.; Poolman, V.; et al. FG-3019, a Human Monoclonal Antibody to CTGF, with Gemcitabine/Erlotinib in Patients with Locally Advanced or Metastatic Pancreatic Ductal Adenocarcinoma. JCO 2013, 31, 213, doi:10.1200/jco.2013.31.4_suppl.213.
- Finger, C.; Cheng, C.-F.; Williams, T.R.; Rankin, E.B.; Bedogni, B.; Tachiki, L.; Spong, S.; Giaccia, A.J.; Powell, M.B. CTGF Is a Therapeutic Target for Metastatic Melanoma. Oncogene 2014, 33, 1093–1100, doi:10.1038/onc.2013.47.
- Neesse, ; Frese, K.K.; Bapiro, T.E.; Nakagawa, T.; Sternlicht, M.D.; Seeley, T.W.; Pilarsky, C.; Jodrell, D.I.; Spong, S.M.; Tuveson, D.A. CTGF Antagonism with MAb FG-3019 Enhances Chemotherapy Response without Increasing Drug Delivery in Murine Ductal Pancreas Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12325–12330, doi:10.1073/pnas.1300415110.
- Abbott, M.; Bortolotto, C.; Benvenuti, S.; Lancia, A.; Filippi, A.R.; Stella, G.M. Malignant Pleural Mesothelioma: Genetic and Microenviromental Heterogeneity as an Unexpected Reading Frame and Therapeutic Challenge. Cancers 2020, 12, 1186, doi:10.3390/cancers12051186.
- Solinas, ; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073, doi:10.1189/jlb.0609385.
- Carbone, ; Yang, H. Molecular Pathways: Targeting Mechanisms of Asbestos and Erionite Carcinogenesis in Mesothelioma. Clin. Cancer Res. 2012, 18, 598–604, doi:10.1158/1078-0432.CCR-11-2259.
- Sayan, ; Mossman, B.T. The NLRP3 Inflammasome in Pathogenic Particle and Fibre-Associated Lung Inflammation and Diseases. Part. Fibre Toxicol. 2016, 13, 51, doi:10.1186/s12989-016-0162-4.
- Kadariya, ; Menges, C.W.; Talarchek, J.; Cai, K.Q.; Klein-Szanto, A.J.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Cheung, M.; Mossman, B.T.; Shukla, A.; et al. Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev. Res. 2016, 9, 406–414, doi:10.1158/1940-6207.CAPR-15-0347.
- Hamilton, F.; Iyer, L.L.; Holian, A. Asbestos Induces Apoptosis in Human Alveolar Macrophages. Am. J. Physiol. 1996, 271, L813–L819, doi:10.1152/ajplung.1996.271.5.L813.
- Jube, ; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer Cell Secretion of the DAMP Protein HMGB1 Supports Progression in Malignant Mesothelioma. Cancer Res. 2012, 72, 3290–3301, doi:10.1158/0008-5472.CAN-11-3481.
- Tabata, ; Shibata, E.; Tabata, R.; Kanemura, S.; Mikami, K.; Nogi, Y.; Masachika, E.; Nishizaki, T.; Nakano, T. Serum HMGB1 as a Prognostic Marker for Malignant Pleural Mesothelioma. BMC Cancer 2013, 13, 205, doi:10.1186/1471-2407-13-205.
- Maroso, ; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like Receptor 4 and High-Mobility Group Box-1 Are Involved in Ictogenesis and Can Be Targeted to Reduce Seizures. Nat. Med. 2010, 16, 413–419, doi:10.1038/nm.2127.
- Horio, ; Minami, T.; Kitai, H.; Ishigaki, H.; Higashiguchi, Y.; Kondo, N.; Hirota, S.; Kitajima, K.; Nakajima, Y.; Koda, Y.; et al. Tumor-Associated Macrophage-Derived Inflammatory Cytokine Enhances Malignant Potential of Malignant Pleural Mesothelioma. Cancer Sci. 2020, 111, 2895–2906, doi:10.1111/cas.14523.
- Marcq, ; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Prognostic and Predictive Aspects of the Tumor Immune Microenvironment and Immune Checkpoints in Malignant Pleural Mesothelioma. Oncoimmunology 2017, 6, e1261241, doi:10.1080/2162402X.2016.1261241.
- Burt, M.; Rodig, S.J.; Tilleman, T.R.; Elbardissi, A.W.; Bueno, R.; Sugarbaker, D.J. Circulating and Tumor-Infiltrating Myeloid Cells Predict Survival in Human Pleural Mesothelioma. Cancer 2011, 117, 5234–5244, doi:10.1002/cncr.26143.
- Cornelissen, ; Lievense, L.A.; Robertus, J.-L.; Hendriks, R.W.; Hoogsteden, H.C.; Hegmans, J.P.J.J.; Aerts, J.G.J.V. Intratumoral Macrophage Phenotype and CD8+ T Lymphocytes as Potential Tools to Predict Local Tumor Outgrowth at the Intervention Site in Malignant Pleural Mesothelioma. Lung Cancer 2015, 88, 332–337, doi:10.1016/j.lungcan.2015.03.013.
- Chu, J.; van Zandwijk, N.; Rasko, J.E.J. The Immune Microenvironment in Mesothelioma: Mechanisms of Resistance to Immunotherapy. Front. Oncol. 2019, 9, 1366, doi:10.3389/fonc.2019.01366.
- Van Acker, H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892, doi:10.3389/fimmu.2017.00892.
- Alay, ; Cordero, D.; Hijazo-Pechero, S.; Aliagas, E.; Lopez-Doriga, A.; Marín, R.; Palmero, R.; Llatjós, R.; Escobar, I.; Ramos, R.; et al. Integrative Transcriptome Analysis of Malignant Pleural Mesothelioma Reveals a Clinically Relevant Immune-Based Classification. J. Immunother. Cancer 2021, 9, e001601, doi:10.1136/jitc-2020-001601.
- DeLong, ; Carroll, R.G.; Henry, A.C.; Tanaka, T.; Ahmad, S.; Leibowitz, M.S.; Sterman, D.H.; June, C.H.; Albelda, S.M.; Vonderheide, R.H. Regulatory T Cells and Cytokines in Malignant Pleural Effusions Secondary to Mesothelioma and Carcinoma. Cancer Biol. Ther. 2005, 4, 342–346, doi:10.4161/cbt.4.3.1644.
- Thapa, ; Salcedo, A.; Lin, X.; Walkiewicz, M.; Murone, C.; Ameratunga, M.; Asadi, K.; Deb, S.; Barnett, S.A.; Knight, S.; et al. The Immune Microenvironment, Genome-Wide Copy Number Aberrations, and Survival in Mesothelioma. J. Thorac. Oncol. 2017, 12, 850–859, doi:10.1016/j.jtho.2017.02.013.
- Murthy, ; Ekeke, C.N.; Russell, K.L.; Butler, S.C.; Wang, Y.; Luketich, J.D.; Soloff, A.C.; Dhupar, R.; Lotze, M.T. Making Cold Malignant Pleural Effusions Hot: Driving Novel Immunotherapies. OncoImmunology 2019, 8, e1554969, doi:10.1080/2162402X.2018.1554969.
- Yamada, ; Oizumi, S.; Kikuchi, E.; Shinagawa, N.; Konishi-Sakakibara, J.; Ishimine, A.; Aoe, K.; Gemba, K.; Kishimoto, T.; Torigoe, T.; et al. CD8+ Tumor-Infiltrating Lymphocytes Predict Favorable Prognosis in Malignant Pleural Mesothelioma after Resection. Cancer Immunol. Immunother. 2010, 59, 1543–1549, doi:10.1007/s00262-010-0881-6.
- Chee, J.; Lopez, M.; Mellows, T.; Gankande, S.; Moutasim, K.A.; Harris, S.; Clarke, J.; Vijayanand, P.; Thomas, G.J.; Ottensmeier, C.H. Evaluating the Effect of Immune Cells on the Outcome of Patients with Mesothelioma. Br. J. Cancer 2017, 117, 1341–1348, doi:10.1038/bjc.2017.269.
- Anraku, ; Cunningham, K.S.; Yun, Z.; Tsao, M.-S.; Zhang, L.; Keshavjee, S.; Johnston, M.R.; de Perrot, M. Impact of Tumor-Infiltrating T Cells on Survival in Patients with Malignant Pleural Mesothelioma. J. Thorac. Cardiovasc. Surg. 2008, 135, 823–829, doi:10.1016/j.jtcvs.2007.10.026.
- Pasello, ; Zago, G.; Lunardi, F.; Urso, L.; Kern, I.; Vlacic, G.; Grosso, F.; Mencoboni, M.; Ceresoli, G.L.; Schiavon, M.; et al. Malignant Pleural Mesothelioma Immune Microenvironment and Checkpoint Expression: Correlation with Clinical–Pathological Features and Intratumor Heterogeneity over Time. Ann. Oncol. 2018, 29, 1258–1265, doi:10.1093/annonc/mdy086.
- Salaroglio, C.; Kopecka, J.; Napoli, F.; Pradotto, M.; Maletta, F.; Costardi, L.; Gagliasso, M.; Milosevic, V.; Ananthanarayanan, P.; Bironzo, P.; et al. Potential Diagnostic and Prognostic Role of Microenvironment in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2019, 14, 1458–1471, doi:10.1016/j.jtho.2019.03.029.
- Fusco, ; Vaira, V.; Righi, I.; Sajjadi, E.; Venetis, K.; Lopez, G.; Cattaneo, M.; Castellani, M.; Rosso, L.; Nosotti, M.; et al. Characterization of the Immune Microenvironment in Malignant Pleural Mesothelioma Reveals Prognostic Subgroups of Patients. Lung Cancer 2020, 150, 53–61, doi:10.1016/j.lungcan.2020.09.026.
- Greten, F.; Manns, M.P.; Korangy, F. Myeloid Derived Suppressor Cells in Human Diseases. Int. Immunopharmacol. 2011, 11, 802–807, doi:10.1016/j.intimp.2011.01.003.
- Mandruzzato, ; Solito, S.; Falisi, E.; Francescato, S.; Chiarion-Sileni, V.; Mocellin, S.; Zanon, A.; Rossi, C.R.; Nitti, D.; Bronte, V.; et al. IL4Ralpha+ Myeloid-Derived Suppressor Cell Expansion in Cancer Patients. J. Immunol. 2009, 182, 6562–6568, doi:10.4049/jimmunol.0803831.
- Burt, M.; Bader, A.; Winter, D.; Rodig, S.J.; Bueno, R.; Sugarbaker, D.J. Expression of Interleukin-4 Receptor Alpha in Human Pleural Mesothelioma Is Associated with Poor Survival and Promotion of Tumor Inflammation. Clin. Cancer Res. 2012, 18, 1568–1577, doi:10.1158/1078-0432.CCR-11-1808.
- Minnema-Luiting, ; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041, doi:10.3390/ijms19041041.
- Khanna, ; Graef, S.; Mussai, F.; Thomas, A.; Wali, N.; Yenidunya, B.G.; Yuan, C.; Morrow, B.; Zhang, J.; Korangy, F.; et al. Tumor-Derived GM-CSF Promotes Granulocyte Immunosuppression in Mesothelioma Patients. Clin. Cancer Res. 2018, 24, 2859–2872, doi:10.1158/1078-0432.CCR-17-3757.
- Upham, W.; Xi, Y. Dendritic Cells in Human Lung Disease: Recent Advances. Chest 2017, 151, 668–673, doi:10.1016/j.chest.2016.09.030.
- Guilliams, ; Lambrecht, B.N.; Hammad, H. Division of Labor between Lung Dendritic Cells and Macrophages in the Defense against Pulmonary Infections. Mucosal Immunol. 2013, 6, 464–473, doi:10.1038/mi.2013.14.
- Kopf, ; Schneider, C.; Nobs, S.P. The Development and Function of Lung-Resident Macrophages and Dendritic Cells. Nat. Immunol. 2015, 16, 36–44, doi:10.1038/ni.3052.
- Lynch, P.; Mazzone, S.B.; Rogers, M.J.; Arikkatt, J.J.; Loh, Z.; Pritchard, A.L.; Upham, J.W.; Phipps, S. The Plasmacytoid Dendritic Cell: At the Cross-Roads in Asthma. Eur. Respir. J. 2014, 43, 264–275, doi:10.1183/09031936.00203412.
- Mitchell, ; Chintala, S.; Dey, M. Plasmacytoid Dendritic Cell in Immunity and Cancer. J. Neuroimmunol. 2018, 322, 63–73, doi:10.1016/j.jneuroim.2018.06.012.
- Gardner, K.; Mamotte, C.D.S.; Patel, P.; Yeoh, T.L.; Jackaman, C.; Nelson, D.J. Mesothelioma Tumor Cells Modulate Dendritic Cell Lipid Content, Phenotype and Function. PLoS ONE 2015, 10, e0123563, doi:10.1371/journal.pone.0123563.
- Jackaman, ; Cornwall, S.; Graham, P.T.; Nelson, D.J. CD40-Activated B Cells Contribute to Mesothelioma Tumor Regression. Immunol. Cell Biol. 2011, 89, 255–267, doi:10.1038/icb.2010.88.
- Vonderheide, H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58, doi:10.1146/annurev-med-062518-045435.
- Hegde, S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874, doi:10.1158/1078-0432.CCR-15-1507.
- Hu, I.; Ghafoor, A.; Sengupta, M.; Hassan, R. Malignant Mesothelioma: Advances in Immune Checkpoint Inhibitor and Mesothelin-Targeted Therapies. Cancer 2021, 127, 1010–1020, doi:10.1002/cncr.33433.
- Paver, C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.-S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed Death Ligand-1 (PD-L1) as a Predictive Marker for Immunotherapy in Solid Tumours: A Guide to Immunohistochemistry Implementation and Interpretation. Pathology 2021, 53, 141–156, doi:10.1016/j.pathol.2020.10.007.
- Akinleye, ; Rasool, Z. Immune Checkpoint Inhibitors of PD-L1 as Cancer Therapeutics. J. Hematol. Oncol. 2019, 12, 92, doi:10.1186/s13045-019-0779-5.
- McCambridge, J.; Napolitano, A.; Mansfield, A.S.; Fennell, D.A.; Sekido, Y.; Nowak, A.K.; Reungwetwattana, T.; Mao, W.; Pass, H.I.; Carbone, M.; et al. Progress in the Management of Malignant Pleural Mesothelioma in 2017. J. Thorac. Oncol. 2018, 13, 606–623, doi:10.1016/j.jtho.2018.02.021.
- Scherpereel, ; Wallyn, F.; Albelda, S.M.; Munck, C. Novel Therapies for Malignant Pleural Mesothelioma. Lancet Oncol. 2018, 19, e161–e172, doi:10.1016/S1470-2045(18)30100-1.
- Jin, ; Gu, W.; Li, X.; Xie, L.; Wang, L.; Chen, Z. PD-L1 and Prognosis in Patients with Malignant Pleural Mesothelioma: A Meta-Analysis and Bioinformatics Study. Adv. Med. Oncol. 2020, 12, 1758835920962362, doi:10.1177/1758835920962362.
- Seaman, ; Zhu, Z.; Saha, S.; Zhang, X.M.; Yang, M.Y.; Hilton, M.B.; Morris, K.; Szot, C.; Morris, H.; Swing, D.A.; et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017, 31, 501–515.e8, doi:10.1016/j.ccell.2017.03.005.
- Matsumura, ; Kajino, K.; Abe, M.; Ohtsuji, N.; Saeki, H.; Hlaing, M.T.; Hino, O. Expression Status of PD-L1 and B7-H3 in Mesothelioma. Pathol. Int. 2020, 70, 999–1008, doi:10.1111/pin.13028.
- Chen, ; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64, doi:10.1186/s13045-018-0605-5.
- Cortes-Dericks, ; Schmid, R.A. CD44 and Its Ligand Hyaluronan as Potential Biomarkers in Malignant Pleural Mesothelioma: Evidence and Perspectives. Respir. Res. 2017, 18, 58, doi:10.1186/s12931-017-0546-5.
- Ohno, ; Shingyoku, S.; Miyake, S.; Tanaka, A.; Fudesaka, S.; Shimizu, Y.; Yoshifuji, A.; Yamawaki, Y.; Yoshida, S.; Tanaka, S.; et al. Differential Regulation of the Sphere Formation and Maintenance of Cancer-Initiating Cells of Malignant Mesothelioma via CD44 and ALK4 Signaling Pathways. Oncogene 2018, 37, 6357–6367, doi:10.1038/s41388-018-0405-y.
- Muller, ; Victoria Lai, W.; Adusumilli, P.S.; Desmeules, P.; Frosina, D.; Jungbluth, A.; Ni, A.; Eguchi, T.; Travis, W.D.; Ladanyi, M.; et al. V-Domain Ig-Containing Suppressor of T-Cell Activation (VISTA), a Potentially Targetable Immune Checkpoint Molecule, Is Highly Expressed in Epithelioid Malignant Pleural Mesothelioma. Mod. Pathol. 2020, 33, 303–311, doi:10.1038/s41379-019-0364-z.
- Galon, ; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218, doi:10.1038/s41573-018-0007-y.
- Sen, ; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-Cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661, doi:10.1158/2159-8290.CD-18-1020.
- Alcala, ; Mangiante, L.; Le-Stang, N.; Gustafson, C.E.; Boyault, S.; Damiola, F.; Alcala, K.; Brevet, M.; Thivolet-Bejui, F.; Blanc-Fournier, C.; et al. Redefining Malignant Pleural Mesothelioma Types as a Continuum Uncovers Immune-Vascular Interactions. EBioMedicine 2019, 48, 191–202, doi:10.1016/j.ebiom.2019.09.003.
- Sekido, Molecular Pathogenesis of Malignant Mesothelioma. Carcinogenesis 2013, 34, 1413–1419, doi:10.1093/carcin/bgt166.
- Strizzi, ; Catalano, A.; Vianale, G.; Orecchia, S.; Casalini, A.; Tassi, G.; Puntoni, R.; Mutti, L.; Procopio, A. Vascular Endothelial Growth Factor Is an Autocrine Growth Factor in Human Malignant Mesothelioma. J. Pathol. 2001, 193, 468–475, doi:10.1002/path.824.
- Ellis, M.; Hicklin, D.J. VEGF-Targeted Therapy: Mechanisms of Anti-Tumour Activity. Nat. Rev. Cancer 2008, 8, 579–591, doi:10.1038/nrc2403.
- König, -E.; Tolnay, E.; Wiethege, T.; Müller, K.-M. Expression of Vascular Endothelial Growth Factor in Diffuse Malignant Pleural Mesothelioma. Virchows Arch. 1999, 435, 8–12, doi:10.1007/s004280050388.
- Ohta, ; Shridhar, V.; Bright, R.K.; Kalemkerian, G.P.; Du, W.; Carbone, M.; Watanabe, Y.; Pass, H.I. VEGF and VEGF Type C Play an Important Role in Angiogenesis and Lymphangiogenesis in Human Malignant Mesothelioma Tumours. Br. J. Cancer 1999, 81, 54–61, doi:10.1038/sj.bjc.6690650.
- Linder, ; Linder, S.; Munck-Wikland, E.; Strander, H. Independent Expression of Serum Vascular Endothelial Growth Factor (VEGF) and Basic Fibroblast Growth Factor (BFGF) in Patients with Carcinoma and Sarcoma. Anticancer Res. 1998, 18, 2063–2068.
- Antony, B.; Hott, J.W.; Godbey, S.W.; Holm, K. Angiogenesis in Mesotheliomas. Role of Mesothelial Cell Derived IL-8. Chest 1996, 109, 21S–22S, doi:10.1378/chest.109.3_supplement.21s.
- Nowak, K.; Brosseau, S.; Cook, A.; Zalcman, G. Antiangiogeneic Strategies in Mesothelioma. Front. Oncol. 2020, 10, 126, doi:10.3389/fonc.2020.00126.
- De Marinis, ; Bria, E.; Ciardiello, F.; Crinò, L.; Douillard, J.Y.; Griesinger, F.; Lambrechts, D.; Perol, M.; Ramalingam, S.S.; Smit, E.F.; et al. International Experts Panel Meeting of the Italian Association of Thoracic Oncology on Antiangiogenetic Drugs for Non-Small Cell Lung Cancer: Realities and Hopes. J. Thorac. Oncol. 2016, 11, 1153–1169, doi:10.1016/j.jtho.2016.03.015.
- Nowak, ; Grosso, F.; Steele, N.; Novello, S.; Popat, S.; Greillier, L.; John, T.; Leighl, N.; Reck, M.; Pavlakis, N.; et al. MA 19.03 Nintedanib + Pemetrexed/Cisplatin in Malignant Pleural Mesothelioma (MPM): Phase II Biomarker Data from the LUME-Meso Study. J. Thorac. Oncol. 2017, 12, S1884, doi:10.1016/j.jtho.2017.09.636.
- Chia, L.; Russell, P.; Asadi, K.; Thapa, B.; Gebski, V.; Murone, C.; Walkiewicz, M.; Eriksson, U.; Scott, A.M.; John, T. Analysis of Angiogenic and Stromal Biomarkers in a Large Malignant Mesothelioma Cohort. Lung Cancer 2020, 150, 1–8, doi:10.1016/j.lungcan.2020.09.022.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13112564
