The skin is our largest organ and the outermost protective barrier. Its aging reflects both intrinsic and extrinsic processes resulting from the constant insults it is exposed to. Aging in the skin is accompanied by specific epigenetic modifications, accumulation of senescent cells, reduced cellular proliferation/tissue renewal, altered extracellular matrix, and a proinflammatory environment favoring undesirable conditions, including disease onset. Macrophages (Mφ) are the most abundant immune cell type in the skin and comprise a group of heterogeneous and plastic cells that are key for skin homeostasis and host defense. However, they have also been implicated in orchestrating chronic inflammation during aging. Since Mφ are related to innate and adaptive immunity, it is possible that age-modified skin Mφ promote adaptive immunity exacerbation and exhaustion, favoring the emergence of proinflammatory pathologies, such as skin cancer.
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Aging is a time-dependent progressive accumulation of significant cellular and tissue changes, including physiological, structural, and functional changes, leading to functional disorders and increased vulnerability to death [
1]. This process is associated with molecular events such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication, which can be termed “hallmarks of aging” [
2,
3].
The skin is our largest organ and constitutes a protective barrier that prevents excessive water loss and the entry of harmful substances and pathogens from the environment. Its aging reflects both intrinsic (or chronological) and extrinsic (such as radiation and pollution exposure) aging processes at the molecular and phenotypic levels [
4]. Skin aging is a process accompanied by changes that alter the local microenvironment, such as weakening of the skin barrier and the accumulation of stressed and senescent cells, both of which foster inflammation through the invasion/release of Pathogen- and Damage-Associated Molecular Patterns [
5]. The consequences of such an altered microenvironment include the promotion of the senescence-associated secretory phenotype (SASP), compromising tissue renewal and function, altered cellular interactions [
6], and chronic low-grade inflammation [
7]. This sterile inflammatory state, termed inflammaging, develops in several organs with advanced age and is associated with persistent inflammation that ultimately exhausts the skin’s defense system [
5,
8].
Macrophages (Mφ), a group of heterogeneous and plastic cells, play a central role in tissue homeostasis and repair, as well as host defense [
9]. In the skin, Mφ can be found in different layers, being classified as recruited Mφ originating from monocytes following a recruitment process started by tissue injury, or as tissue-resident macrophages (TRM), which are derived from both adult and embryonic progenitors [
10]. In the interfollicular epidermis, there are the Langerhans cells (LC), which can migrate to the lymph nodes to present antigens, being related to antimicrobial immunity, immune surveillance, and contact hypersensitivity [
11]. Due to the shared characteristics with dendritic cells (DCs), LC have long been classified as such [
12,
13]. Nevertheless, after further ontogeny studies have demonstrated that LC arise from embryonic precursors and are maintained within the epidermis by local self-renewal under steady-state conditions, LC are currently considered a specialized subset of TRM [
14]. Mφ located in the dermis, on the other hand, are called dermal Mφ and are associated with tissue repair and clearance [
15].
To exert such a variety of functions, Mφ may acquire different phenotypes in response to various stimuli. In this sense, based on in vitro assays, Mφ have been divided into two groups based on their polarization phenotypes: M1 and M2. Classically activated Mφ are deemed as M1 and constitute catabolic, proinflammatory cells that are involved in antimicrobial host defense. M2, or alternatively activated Mφ, are anabolic cells with anti-inflammatory and tissue repair properties [
16]. However, mainly due to recent advances in single-cell RNA sequencing (scRNA-Seq), it is now clear that such a dichotomy does not accurately represent Mφ in vivo but represents the extremes of a wide range of continuous phenotypes which have been reported [
17,
18].
The aging process has a great impact on Mφ, including alterations in Mφ metabolic and immune function, impacting the Mφ capability of clearance and immunosurveillance, constituting an important aspect of immunosenescence [
19]. In fact, old Mφ in a mice model were characterized with a senescent, proinflammatory profile [
20], associated with increased oxidative stress, compromised antioxidant defenses, and impaired function [
21].
Interestingly, the number of LC in the skin and their capacity to migrate to the lymph node and stimulate T cells seems to be reduced in aged subjects compared to young ones [
22]. In aged mice, the same process is observed, accompanied by a decline in LC maturation, but not in LC proliferation and survival levels, suggesting either a deficiency in bone marrow-derived LC progenitors or the generation of progenitors that are less responsive to chemokine and cytokine signals [
22]. The same study has also described a higher level of phagocytosis in Mφ from older mice [
22], which is probably a result of an age-related Mφ hyperfunction, since during the aging process the skin barrier weakens, favoring the pathogen’s invasion and stressed and senescent cells that should normally be eliminated are accumulated [
23].
Mφ are considered as gatekeepers of tissue homeostasis and integrity, constituting primary inflammatory cytokine producers, as well as initiators and regulators of inflammation, and representing one of the main cellular players in adaptive immunity exacerbation and exhaustion during aging [
24,
25]. With that being said, it is possible to consider Mφ as important players in the promotion of chronic proinflammatory-associated pathologies, such as psoriasis [
26,
27], rosacea [
28,
29], vitiligo [
30,
31], and skin cancer [
32,
33].
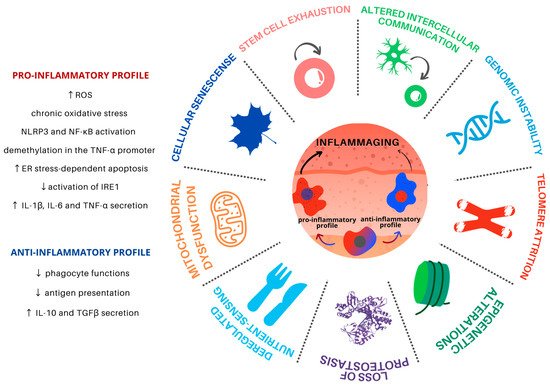
2. Hallmarks of Aging and Macrophages
Aging is a progressive and common process for all cells and tissues and can be caused by both intracellular and extracellular factors. It leads to organismal dysfunction on multiple levels, the main underlying processes being identified as the hallmarks of aging (). Such hallmarks are interconnected and converge to tissue inflammation and dysfunction [
5].
Figure 1. Hallmarks of aging in Mφ in the skin microenvironment. Skin inflammaging is fostered by different yet interconnected and synergistic aging hallmarks. Mφ are plastic cells that play a pivotal role in the immune system and have been associated with the persistent chronic inflammation levels found in aged skin. Skin inflammaging is characterized by a shift towards pro-inflammatory Mφ phenotypes, which promote further tissue inflammation in the skin microenvironment through the secretion of pro-inflammatory cytokines, activation of important inflammatory pathways, and increased oxidative stress. Chronic low-grade oxidative-inflammatory stress during the aging process is a key factor that stimulates a vicious cycle, contributing to age-associated disease onset. At the same time, the reduction of Mφ with an anti-inflammatory phenotype contributes to the decrease in antigen presentation and phagocytosis, contributing to tissue homeostasis disturbance.
2.1. Genomic Instability
DNA damage accumulation is expected to occur with aging and accumulates as a result of many endogenous and exogenous factors. Genomic instability in the aging process can be associated with somatic mutations, copy-number alterations, and chromosome abnormalities for nuclear as well as mitochondrial DNA (mtDNA) [
2]. Such DNA alterations may affect essential genes and transcriptional pathways, resulting in dysfunctional cells.
In the literature, the causes of genomic instability in Mφ are scarce and one work points out that it can be induced by pathogens such as
Mycobacterium tuberculosis [
34].
M. tuberculosis is the causative agent of tuberculosis with a pathological outcome associated with the formation of granulomas. The granulomas formed in the development of several chronic diseases (due to persistent inflammatory stimuli) can modulate molecular programs, which are involved in TRM differentiation and relate to clinical outcomes [
35] and DNA damage [
36].
Since Mφ are the most abundant immune cell type in the skin, and this tissue is directly exposed to several environmental factors, such as UV radiation, genomic instability could be a central hallmark of aging in aged Mφ; after all, numerous DNA injuries can lead to accumulated damage and altered Mφ function in this tissue [
37]. This in turn can lead to altered gene expression of molecules such as cytokines, MHC class II, transcription factors, an exacerbated production of reactive oxygen species (ROS) [
15], and NF-κB signaling in response to DNA damage [
38], rendering Mφ more susceptible to apoptosis, impairing their phagocytosis function [
39], and contributing to inflammaging [
40].
2.2. Telomere Attrition
Telomeres protect the ends of chromosomes from degradation and abnormal recombination. Considered a primary hallmark of aging, telomere attrition causes the loss of chromosome protective structures as they gradually get shorter [
2]. This shortening process has also been closely connected with inflammation [
41,
42].
Comparing young and old mice, Kang and colleagues (2018) observed that the shortening of telomeres in Mφ leads to increased ROS production, the same phenotype observed for genomic instability (see
Section 2.1). Furthermore, in their experiment, knockout mice for Telomerase RNA Component (
Terc−/−), a gene that encodes for the RNA that serves as a template for the telomere repeats, showed telomere dysfunction in Mφ, which is associated with hyper inflammation and mitochondrial abnormality, followed by oxidative stress with hyperactivation of the Nod-like receptor protein 3 (NLRP3) inflammasome (see
Section 2.6) [
43].
The increase in oxidative stress can induce DNA breakdown, which can lead to mutations that may explain most of the changes described in the aged Mφ. The main inflammatory signaling pathway, NF-κB, regulates the maintenance of telomeres and telomerase activity [
44], just as the latter regulate NF-κB activity [
45]. This relationship leads to a defective autophagic response and overexpression of inflammatory cytokines, such as TNF-α, IL-6, and IFN in circulating Mφ [
46].
2.3. Epigenetic Alterations
Epigenetic alterations involve changes in DNA methylation (DNAm) patterns, post-transcriptional modification of histones, and chromatin remodeling [
2,
47]. These aging-induced epigenetic changes in Mφ are mainly responsible for controlling the inflammatory profile and cell differentiation [
48,
49].
DNAm undergoes predictable time-dependent modifications across CpG islands and is influenced by both intrinsic and extrinsic processes. Molecular clocks have been developed in order to calculate the “biological age” of biological samples using methylome data [
50,
51], including a skin-specific Molecular Clock [
51]. Age-associated epigenetic remodeling involves highly localized gain/loss of DNAm at the binding sites of transcription factors associated with the monocyte-macrophage differentiation process [
52]. Despite a major lack of comprehension regarding the cause or effect role of epigenetic changes and aging phenotypes, recent studies have shed light on at least a few events that connect epigenetic changes in Mφ and age-related phenotypes, such as inflammation and differentiation.
For instance, aging-associated changes in DNAm, particularly the demethylation in the tumor necrosis factor (TNF-α) promoter, a cytokine predominantly produced by Mφ, revealed a possible link between inflammation, Mφ, and chronic age-related diseases. The promoter demethylation has been described to occur in peripheral blood leukocytes and Mφ of aging subjects and is accompanied by a reduction of TNF-α reporter gene activity [
53], possibly associated with chronic inflammatory processes.
Other studies have also revealed that protein-3 containing the Jumonji domain (Jmjd-3), a H3K27 demethylase, promotes the induction of Irf-4, and SMYD-3, an H3K4 methyltransferase, of IL-4 and IL-12 [
54,
55]. These histone-modifying proteins play a central role in aging and their activities increase in the course of this process. In Mφ, they contribute to the positive regulation of regenerative and anti-inflammatory profiles [
55,
56].
These epigenetic alterations in the skin microenvironment contribute to inflammaging and can be directly linked to clinical outcomes. For instance, the chronic exposure of Mφ to inflammatory triggers and products of dead or senescent cells (see
Section 2.7) can impose epigenetic changes that cause Mφ’ altered response capacity during skin aging [
52].
2.4. Loss of Proteostasis
Proteostasis is defined as the process of protein homeostasis maintenance and comprises a complex proteostasis network (PN), mainly composed by specialized proteins such as chaperones and cochaperones, translational machinery, the ubiquitin-proteasome system (UPS), and the autophagy machinery [
57]. The PN has the role of controlling protein synthesis, modification, secretion, and degradation. It also reduces misfolded proteins by restoring, removing, or degrading them through the unfolded protein response (UPR) activity to prevent their accumulation in cellular compartments [
58]. The chaperone-mediated autophagy (CMA) is another player in the proteostasis balance. HSPA8 is a central component of CMA and is an abundant protein in Mφ and other immune cells. Together with a co-chaperone complex, HSPA8 recognizes “CMA-targeting recognition motifs” in the targeted protein sequence, unfolding the substrate and delivering it to a protein called lysosome-associated membrane protein 2A (LAMP2A), which internalizes the targeted proteins for subsequent degradation in the lysosomal lumen [
59]. If those mechanisms fail to restore homeostasis, apoptotic pathways may be activated to ensure survival of the organism [
58].
Autophagy is a paramount process in the maintenance of skin homeostasis throughout aging, the consequences of age-related autophagy decay affecting different skin types, including LC. An example describing the consequences of the loss of proteostasis on aged skin is the change of elastin, collagen, and melanin levels [
60,
61,
62] found in wrinkled and hypopigmented skin [
60,
63]. The balance in the composition of those proteins is essential for skin function and health and, interestingly, Mφ play an important role in this context. For instance, Mφ synthesize metalloelastases (e.g., metalloelastase 12) that participate in the elimination of nonfunctional elastin aggregates generated in the skin as a consequence of photoaging [
64,
65].
If on one side, healthy Mφ are important contributors to the maintenance of proteostasis, aged Mφ exhibit diminished inositol-requiring enzyme 1α (IRE1α) activation (a stress sensor that activates UPR) and increased susceptibility to endoplasmic reticulum (ER) stress-dependent apoptosis [
66]. During high levels of ER stress, UPR activates IRE1α, which in turn assists the alternative splicing of X-box binding protein 1 (XBP1) mRNA [
67]. After its activation, the transcription factor XBP1 induces the expression of cytokines such as pro–IL-1β [
68]. However, it has been shown that Toll-like Receptors (TLR) in mice and human Mφ can directly activate XBP1, without UPR mediation, or even in synergy with ER stress [
67], leading to the splicing of XBP1 and activation of a sustained proinflammatory environment by IL-1β, IL-6, and TNF [
67,
69].
In fact, it has been shown that the inflammasome is activated in the context of excessive misfolded protein accumulation, which is exacerbated in autophagy- or p62 (sequestosome 1)-deficient Mφ [
70,
71]. In addition, ER stress can also be transferred from neighboring parenchymal cells to TRMs by upregulating the splicing of UPR components, such as
Grp78,
Gadd34,
Chop, and
Xbp-1 [
72,
73]. This phenomenon of a “transmissible” ER stress state is mediated by the production of IL-4, IL-10, and by apoptotic bodies from stressed cells [
72]. Therefore, the age-related loss of proteostasis in the skin affects Mφ and seems to contribute to the local inflammaging phenotype.
2.5. Deregulated Nutrient Sensing
Aging directly affects the sensors and molecular targets of nutrients and fluid homeostatic regulation [
74]. One of the underlying mechanisms by which aging promotes deregulated nutrient sensing is by promoting disturbed insulin sensitivity, which compromises the capacity of some tissues to uptake and metabolize nutrients [
75].
Importantly, nutrient sensing components have been directly linked to longevity, including the mammalian targets of rapamycin (mTOR) and AMP-dependent protein kinase (AMPK), the two major components of nutrient sensing and metabolic regulation. AMPK has an inhibitory effect on mTOR signaling, which is activated during nutrient starvation, leading to a rise in the AMP:ATP ratio [
76]. In association with nicotinamide adenine dinucleotide (NAD), high AMP levels activate sirtuins, responsible for insulin signaling pathway and longevity regulation. Consequently, the excess of nutrient availability can promote aging-associated diseases [
77,
78].
Accordingly, the modulation of nutrient sensing signaling influences several immune cell types including Mφ. It has been said that the Mφ immunometabolism influences Mφ polarization and activation, processes which are tightly linked to skin homeostasis and inflammaging. Mφ metabolic signatures have been closely connected with the M1-like and M2-like phenotypes; the M1-like Mφ heavily relying on glycolysis, and the M2-like Mφ being more dependent on oxidative phosphorylation [
79]. During the aging process, Mφ function and phenotypes are disturbed due to many factors, including nutrient sensing dysregulation and the installation of a chronic low-grade inflammation environment in the tissue. In this sense, deregulated nutrient sensing can increase Mφ glycolysis and suppress the oxidative phosphorylation (via attenuation of IL-4-induced anti-inflammatory responses), favoring the accumulation of M1-like Mφ in the aged skin [
80,
81,
82,
83]. The presence of M2-like profile is therefore reduced, causing skin damage and promoting the progression of age-associated diseases [
84,
85,
86].
Furthermore, FOXO and mTOR are targets of the insulin and insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway and are influenced by nutrient status, altering tissue homeostasis, and inflammation [
87,
88]. In a very elegant experiment, the skin of mice lacking both the insulin and IGF-1 receptor in myeloid cells was enriched in noninflammatory Mφ phenotype after the induction of dermatitis. When compared to controls, it showed evidence of a proinflammatory IR/IGF-1R-dependent pathway and a connection between cutaneous inflammatory responses and diseases such as insulin-resistant diabetes mellitus type 2 [
89]. In addition, SASP has been highly associated with Mφ inflammatory factors in conditions of hyperglycaemia, contributing to the fueling of low-grade inflammation in diabetes [
90]. Under nutrient starvation, FOXO1 migrates to the nucleus after phosphorylation and seems to stimulate proinflammatory TLR4 signaling and IL-8β production in Mφ. FOXO1 migration also stimulates the expression of the anti-inflammatory cytokine IL-10 in M2-like cells [
91], supporting the phenotypic development of aging Mφ in distinct directions [
19].
Bone marrow-derived Mφ have also shown an increasing expression of growth hormone receptor (GH-R) and GH-R-dependent induction of inflammatory components in aged mice. Current evidence suggests that the downregulation of NLRP3 inflammasome in Mφ by GH-R is capable of maintaining immune system homeostasis and extending health- and lifespan [
92].
Since nutrient sensing signaling pathways can be pharmacologically modulated and are closely linked to inflammation, interesting observations could be made regarding the manipulation of Mφ phenotypes in the skin. For instance, the modulation of AMPK/mTOR/NLRP3 inflammasome signaling using Metformin revealed that the drug treatment promoted reduced NLRP3 signaling and promoted the regenerative M2-like phenotype in skin Mφ, paving a way to re-establish skin Mφ equilibrium [
93]. Several other anti-inflammatory drugs target immunometabolism and may also contribute in this sense, as revised by [
94].
2.6. Mitochondrial Dysfunction
Mitochondrial production of ROS is crucial for the skin’s defense against pathogens [
95]. However, dysfunctional mitochondria in Mφ can generate excessive production of ROS and cause damage to important intracellular structures, including the mtDNA [
22], contributing to defective apoptosis and activation of inflammasomes [
96]. Aged Mφ present mitochondrial dysfunction associated with decreased ATP production, reduction of mitochondrial membrane potential (ΔΨm), and increased oxidative stress, as well as depreciated antioxidant defense response that can impair Mφ functions and lead to senescence [
97]. For instance, during lung
Streptococcus pneumoniae infections, impaired mitochondrial function of aged Mφ increases lung pathology and oxidative stress [
98].
The activity of the oxidative phosphorylation system is also altered in aging. During aerobic respiration, oxygen can be reduced prematurely, generating a high amount of ROS, a process that is exacerbated in senescent cells [
99]. Besides that, Minhas and colleagues (2019) demonstrated the importance of NAD
+ levels to maintain mitochondrial respiration and regulate Mφ phagocytosis in an anti-inflammatory homeostatic state, both in vitro and in vivo. NAD
+ levels can be replenished by de novo synthesis and via the kynurenine pathway. Blockage of de novo NAD
+ synthesis impaired phagocytosis and resolution of inflammation in aged Mφ [
100].
In addition to sensing and cleansing cellular debris, Mφ also detect accumulation of mitochondrial garbage in the cellular microenvironment, leading to a continuous stimulation of these cells and thus their activation [
101] and thus sustaining an environment of chronic low-grade inflammation with production of cytokines and ROS [
97]. ROS accumulation in intracellular microenvironments (not only in the mitochondria) can cause DNA damage in aged tissues [
28]. In the context of the skin, ultraviolet (UV) radiation-induced mtDNA injury also leads to more ROS production, accelerating photoaging [
102]. In photodamaged skin, xanthine oxidase-induced ROS is reported to be the cause of alterations in collagen biosynthesis in cultured human dermal fibroblasts [
103]. Moreover, other enzymatic and non-enzymatic sources of ROS are observed in the skin. Besides mitochondrial ROS production via electron transport chain and UV-induced ROS, there is production of ROS via peroxisomes, ER, and skin cell membranes [
104]. In cultured Mφ, Ives and colleagues (2015) demonstrated that xanthine oxidase (XO) is the major source of ROS [
105]. XO expression and activity has also been shown to be increased in old mice, closely associating with oxidative stress and exacerbated ROS formation [
21].
As extensively revised by Beek and colleagues (2019), there is a tight link between mitochondrial dysfunction and ER in aged Mφ, that results in impaired calcium and redox homeostasis and leads to oxidative stress and activation of several pathways, including the inflammasome. Consequently, a proinflammatory environment is promoted by enhancing IL-1β secretion and nuclear translocation of NF-kB [
19]. Furthermore, it has been proposed that this age-related oxidative-inflammatory stress (“oxi-inflammaging”) occurs in a vicious cycle during aging [
106]. Taken together, it can be expected that age-related ER and oxidative stresses can contribute to an enhanced production of pro-inflammatory cytokines in Mφ and thus to systemic inflammaging, favoring the onset of pathologies.
2.7. Cellular Senescence
Cellular senescence is a cellular state characterized by cell cycle arrest, even under growth-promoting conditions [
2]. Other phenotypes of cellular senescence include apoptosis resistance and SASP [
107]. In the human skin, senescent keratinocytes and fibroblasts accumulate with age and support a feedforward system mainly mediated by SASP to accelerate tissue function decay [
108]. Senescent cells show increased production of proinflammatory cytokines, chemokines, growth factors and metalloproteinases [
109], telomere attrition (see
Section 2.2), epigenetic alterations (see
Section 2.3), loss of proteostasis (see
Section 2.4), and dysfunctional mitochondria (see
Section 2.6), underscoring the many facets of the senescent cell phenotype.
The age-related alterations of immune system elements have been defined as immunosenescence and are characterized by changes in anatomical barriers, lymphoid organs and immune cell function, all of which synergize to result in a systemic organismal low-grade inflammation, deemed inflammaging [
5].
Intrinsic and extrinsic factors of immunosenescence affect both recruited Mφ and TRMs. In homeostatic conditions, activated Mφ clear cell debris [
110], but when senescent cell clearance is not effective, such cells accumulate and intensify SASP, causing several alterations in the local milieu, including Mφ dysfunction [
111]. Using a mouse model, Prattichizzo and colleagues (2018) demonstrated that in hyperglycemic conditions, both cellular senescence and SASP can be induced in Mφ [
90]. In aged tissues, the activated Mφ produces molecules that drive inflammatory response, such as IL-6, matrix metalloproteinases, chemokines and other mediators [
112,
113]. Together, these indicate that dysfunctional Mφ are both a result of dysfunctional niches and cellular senescence, in addition to contributing to the maintenance of low-grade tissue inflammation.
2.8. Stem Cell Exhaustion
Stem cell exhaustion is a consequence of the sum of several hallmarks of aging mentioned above and is likely one of the main culprits for the loss of tissue regenerative capacity, and consequently organismal aging. Examples of that loss have already been related to immunosenescence [
114,
115].
Skin homeostasis is mainly maintained by two stem cell types: dermal mesenchymal stem cells (dermal MSCs), present in the inner layer of dermis, and epidermal stem cells (ESCs), located in the basal epidermal layer. While ESCs are responsible for epidermal cell renewal, a consequence of the capacity to differentiate into different cell lineages of the skin, such as keratinocytes and melanocytes [
116,
117], dermal MSCs are capable of differentiating into subcutaneous adipocytes, osteoblasts, and chondrocytes [
118,
119]. With aging, a loss of ESC and dermal MSC production and differentiation is observed, with consequent deceleration of skin cell renewal and reduction of skin healing capacity [
119,
120].
Tissue-resident hematopoietic cells, progenitors of Mφ, can self-maintain independently of hematopoietic stem cells (HSC). Mφ derived from yolk-sac are replaced by HSC-derived ones only in a few organs, including epidermis skin layer (LC originating from erythro-myeloid progenitors) [
121]. This statement suggests a more important role is played by the cell’s origin than its tissue location in life span [
14]. As a consequence, it is not surprising that the aged skin tends to have less LC in its composition, decreasing antigen-specific immunity [
122].
Wound repair is a continuous process comprising four phases: hemostasis, inflammatory, proliferative, and remodeling (or resolution) phases [
123]. With the advance in aging, there is a delayed lesion reepithelization and decreased tensile strength [
124,
125,
126]. That may also indicate the importance of the presence of Mφ subpopulations in key moments of wound skin repair [
123]. The M1-like Mφ profile contributes to the early stage of skin healing by promoting an inflammatory response with the production of high levels of proinflammatory cytokines. On the other hand, M2-like Mφ are responsible for the tissue repair process itself, including the regulation of re-vascularisation processes, fibroblast proliferation, and myofibroblast conversion, in addition to collagen production via inhibition of the AMPK/mTOR/NLRP3 inflammasome signaling axis, as discussed in
Section 2.5. [
93]. Immune cells such as Mφ are capable of activating epidermal stem cells for re-epithelialization under the establishment of an inflammatory wound microenvironment [
127].
Through single-cell transcriptomic data analysis, a study was capable of characterizing a dermal subpopulation of Mφ that contributes to local nerve regeneration and axon sprouting after a mechanical injury [
128]. Another study also observed that during skin repair, Mφ are also capable of stimulating the proliferation of adipocyte precursors [
129]. Aged Mφ tend to lose their ability to migrate into wounds, with consequent retention of the Mφ at the dermis and increased tissue damaging release of ROS and proinflammatory cytokines [
130]. In the epidermis, such an excessive proinflammatory microenvironment depletes epidermal stem cells, further contributing to compromised skin healing capacity [
131].
2.9. Altered Intercellular Communication
The age-related changes in intercellular communication have been characterized in the autocrine, paracrine, endocrine, and neuroendocrine levels. The neurohormonal signaling (e.g., renin-angiotensin, adrenergic, insulin-IGF1 signaling) tends to be deregulated in aging as inflammatory reactions increase, immunosurveillance against pathogens and premalignant cells declines, and the composition of the peri- and extracellular environment changes, thereby affecting the mechanical and functional properties of all tissues, including the skin [
2,
132].
Recently, it was observed that mast cells are important in the recruitment of Mφ in aging through the change in the pattern of chemoattractant cytokines [
133]. But there have still been few studies on how these intercellular communications involve Mφ in the skin. Still, it is already known that the age-related changes in intercellular communication are associated with inflammation. The accumulation of tissue damage throughout life, the likelihood of cytokines being secreted by senescent cells, the enhanced activation of the NF-κB transcription factor, and the occurrence of a defective autophagy response all seem to foster the immune system failure [
96,
134].
This entry is adapted from the peer-reviewed paper 10.3390/cells10061323