Natural biopolymers are an interesting resource for edible films production, as they are environmentally friendly packaging materials.
- biopolymers
- edible films and coatings
- dry and wet processes
- mechanical properties
- permeability
1. Introduction
Inferior packaging or its absence causes significant food loss (about 20–25 %) due to microbiological contamination and oxidative processes, which lead to a decrease in the quality of food products and makes them unsuitable for consumption [1].
The development and application of bioactive packaging systems is a relevant field of research. The application of such smart packaging systems is a tool for protecting food from spoilage and reducing the risk of growth of pathogenic microorganisms due to both the creation of a barrier and the action of active components at the border of the product with the packaging [2][3]. The currently used packaging materials are mainly produced from petrochemical products [4][5]. The global problem of environmental pollution makes alternative environmentally friendly and biodegradable polymers be in demand [6].
Every year, the problem of recycling polymer packaging materials becomes more acute [7] due to its accumulation in large quantities, which cause significant harm to the environment [8]. The incineration or pyrolysis of polymer waste, to some extent, solves the problem of their accumulation in landfills, but does not contribute to improving the overall environmental situation [9]. Recycling of polymer waste is more environmentally friendly, but in this case, significant labor and energy costs are required for sorting polymer materials and their subsequent processing [10][11]. It should be noted that the recycling of polymers is carried out a limited number of times, after which the problem of burial or incineration of these materials arises again [12].
Concerning environmental suitability, biopolymers are environmentally friendly packaging materials [13][14]. The main advantage of their use as bioplastics is the closed natural cycle, where the end of one cycle leads to the beginning of the next cycle [6].
Biopolymers can be divided into three main categories depending on the origin and method of production (Figure 1): directly extracted from biomass, synthesized bio-derived monomers, and produced by microorganisms [15]. Polysaccharides and proteins are the most promising biopolymers for the production of packaging materials [16][17]. Proteins are heteropolymers consisting of α-amino acids as monomeric units. The combinations of 20 amino acids to form a protein sequence allow for an almost unlimited number of various polymer chains with different physical and chemical properties. Proteins also contain a large number of functional groups that can be changed enzymatically, chemically, or physically for varying the properties of the films [16]. Polysaccharides are good candidates for replacing oil-based polymers due to their ability to form a film, affinity for paper-based materials, an appropriate barrier to gases and aromas, and good mechanical strength. Moreover, these biopolymers are biodegradable, non-toxic, and are used as a matrix for the inclusion of additives with specific functionality, such as active antimicrobial properties, for example [17].
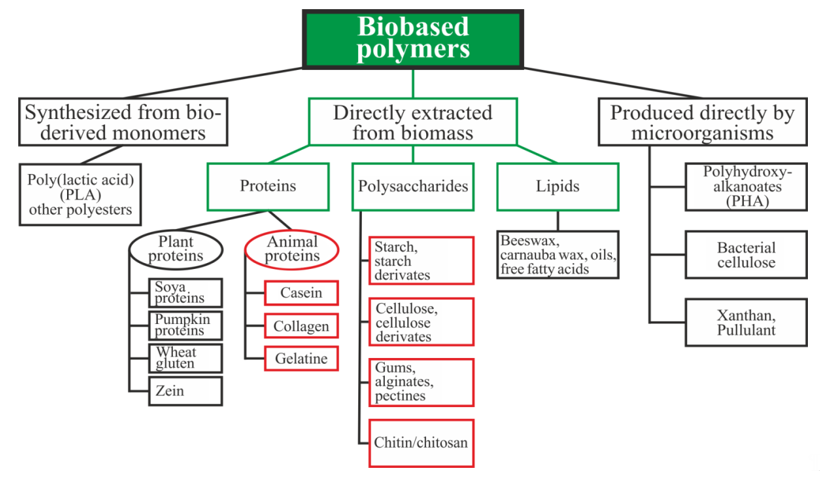
Figure 1. Schematic classification of biopolymer types [15][18][19]. Reproduced with permission from Popović, S.Z. et al., Biopolymers for Food Design; published by Elsevier Inc., 2018.
The prefix "bio" indicates that biopolymers are biodegradable. The word "biodegradable" means that materials can be decomposed by bacteria, fungi, and yeast to the final products of biomass under anaerobic conditions—hydrocarbons and methane [20]. These types of polymers consist of monomers that are covalently connected, forming a chain of the molecule. They are also produced inside the cell as a result of complex metabolic processes. Biopolymers can be used for food packaging as a replacement for oil-based plastics made from petroleum due to their biodegradability, renewability, and wide distribution [21].
Environmental friendliness is a key ideology nowadays. The use of biopolymers from renewable resources could solve global plastic pollution. For many years, researchers have been trying to develop and design packaging materials based on natural biopolymers. However, animal proteins and natural polysaccharides are characterized by some undesirable properties caused by their chemical nature and structure. These disadvantages reduce their competitiveness with oil-based plastics but can be overcome.
2. Biopolymers Used for Food Packaging
Biopolymers are widely implemented and can be used as coatings and films [4][22]. Coating involves the formation of a cover directly on the surface of food products, whereas films are structures that are used separately after formation [23].
Materials based on biopolymers must meet the basic requirements of health safety, mechanical and chemical resistance, and durability [24][25]. Therefore, food packaging should not only be biodegradable, but also functional. Compared to synthetic polymers derived from petroleum, biopolymers have a more complex chemical structure and side chain structure, which provides additional opportunities for the formation of packaging materials with specific characteristics for specific purposes [18][20].
Biopolymers directly extracted from biomass as polysaccharides and animal proteins, which are most often used for the preparation of packaging materials for food products, will be considered in this review [16][26][27].
2.1. Starch
Starch is one of the most readily available polysaccharides on the planet [28]. The plants from which it is obtained grow in almost all temperate climate zones. Corn, wheat, potatoes, and rice are the world leaders: 84 %, 7 %, 4 %, and 1%, respectively [29]. This biopolymer is a mixture of amylose and amylopectin (Figure 2), the ratio of which varies depending on the type of starch.
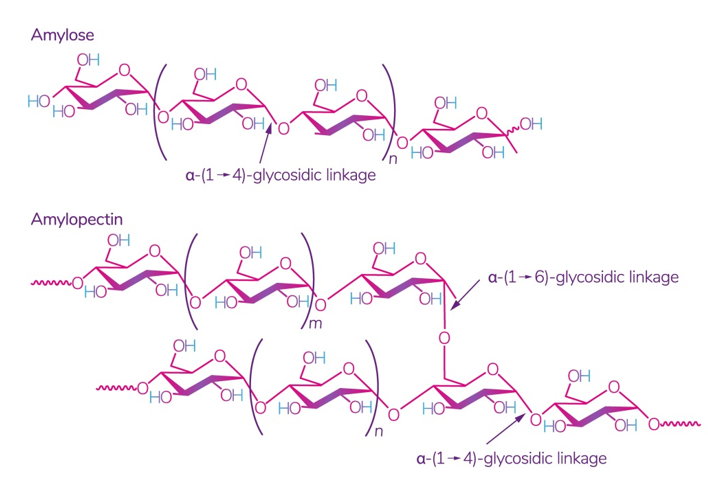
Figure 2. Structure of amylose and amylopectin [30]. Reproduced from Kadokawa, Polymers; published by MDPI, Basel, Switzerland, 2012.
The ratio of amylose:amylopectin varies significantly not only between different plants, but also within a single plant species or plant organ [31]. The conditions and phase of growth also influence this ratio [32][33]. Several studies have shown that a change in this ratio implies a change in the physical and chemical characteristics of starch and its interaction with other molecules, which leads to different swelling capacity [34], solubility in water, microscopic properties [35], and stability and barrier/mechanical properties in starch films [36]. According to some studies, starch with a high content of amylose, for example, from peas, has the best mechanical and gas barrier properties [37]. It was also noted that lentil starch (30% amylose) has a strong tendency to gelatinize at a relatively low concentration compared to corn and potato starch [29].
Cereal (grain) starch is obtained by its physical separation from non-starch components. Various processes of wet grinding of grains have been developed for the production of starch. The main stages of these processes are soaking, grinding, and separation of grain components [38]. Potato starch is extracted from potato tubers in a process called bio-processing: the potatoes are ground, and the contents of the cells are released, including starch and protein [39].
Starch-based polymers have low moisture resistance, which limits their use in packaging. The usual form of natural starch is a crystalline molecular structure that is not flexible [40]. However, an interesting starch derivative is thermoplastic starch (TPS), which is more convenient for films production [41][42], which could be obtained with thermal and physical impacts in the presence of plasticizers [40]. Various physical and chemical reactions are involved in the heat treatment of starch-based polymers, such as water diffusion, granule expansion, gelatinization, melting, crystallization, and extrusion [43].
TPS with improving properties can be used in the field of food packaging since it is economical and available in large quantities. The production of flexible and solid packaging (biofilms, bags, laminated plastic, etc.) is the main sector of TPS application in the food industry [40]. Polymer films derived from starch are biodegradable and have good properties as oxygen barriers. However, the amount of plasticizer, humidity, and amylose content are the limiting factors that determine the mechanical properties of TPS. The type and amount of plasticizer used in the production of thermoplastic starch strongly affect the physical, chemical, and thermal properties of the film [44]. Various structural enhancers, such as microcrystalline cellulose, carboxymethylcellulose, carbon nanotubes, etc., are added to the starch-polymer matrix in order to improve its properties [45]. Various types of such reinforced starch are already successfully used in the packaging of bread, vegetables, and meat products stored in standard conditions [41][46][47].
2.2. Cellulose
Cellulose is the most common natural biopolymer and consists of β-(1–4)-D-glucopyranose monomers (Figure 3) [48]. It is biosynthesized by a number of living organisms, from lower to higher plants, marine animals, bacteria, and fungi [49]. It has been estimated that 1011–1012 tons are synthesized annually by photosynthesis in a fairly pure form, for example, in the seed hairs of the cotton plant, but mainly cellulose combines with lignin and other polysaccharides (hemicelluloses) in the cell wall of woody plants [50]. Although cellulose is primarily found in forests, where wood is the most important source, cellulose-containing materials include agricultural residues, aquatic plants, grasses, and other plant matter [51][52]. Commercial cellulose production is based on harvested sources, such as wood, or on natural sources with high biopolymer content, such as cotton [53]. In contrast with starch, cellulose is a linear polymer without winding and branching [48]. Numerous hydroxyl groups in cellulose form strong hydrogen bonds, which make the material non-fusible. Chemical modification of cellulose is required for the production of flexible materials, which often involves the replacement of hydroxyl groups with acetate or methyl groups (esterification), the purpose of which is to reduce the intensity of the hydrogen bonds [54]. The rate of esterification and type of replacers, as well as the length of the polymer chain, affect the further permeability, mechanical properties, and solubility. Methylcellulose (MC) and carboxymethylcellulose (CMC) are the most common cellulose esters and have good film-forming properties, which allow them to be used as packaging materials for food products [55][56][57][58].
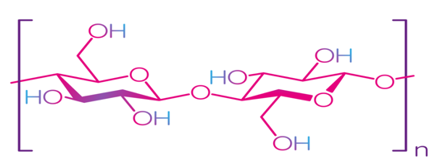
Figure 3. Structure of cellulose [49]. Reproduced with permission from Trache, D. et al., International Journal of Biological Macromolecules; published by Elsevier Ltd., 2016.
Methylcellulose (MC) is formed when one or more hydroxyl groups (-OH) in the anhydroglucose are replaced by a methoxide group (-OCH3). The degree of substitution of MC ranges from 1.4 to 2.0, and it is soluble in cold water [59]. It is reported that MC forms unbreakable, flexible, transparent, tasteless, and non-toxic films that have good barrier properties for oxygen, but bad ones for water vapor [60]. MC has a relatively high tensile strength and elastic modulus, so it demonstrates a reinforcing effect [61] and exhibits improved mechanical properties in mixtures containing proteins, lipids, etc. [62].
Carboxymethylcellulose (CMC) is a cellulose derivative in which some hydroxyl groups of glucopyranose units in cellulose are replaced by carboxymethyl groups. CMC is formed by reaction with chloroacetic acid (ClCH2CO2H) and catalyzed by alkali [63]. The amount of carboxymethyl groups in the cellulose molecule improves the strength of CMC film due to strong intermolecular forces [64]. Initially, CMC was studied as a hydrogel polymer, but it was later discovered that its dry form could be considered a biodegradable alternative to petroleum-based food packaging materials [64]. CMC easily absorbs moisture, dissolves in cold water, and exhibits thermal gelatinization [65]. CMC-based films can be combined with MC, clay, chitosan, etc., which usually improves the mechanical properties of the film: the tensile strength and elastic modulus increase, while the strain at break of the films is reduced [65]. It was noted that such a plasticizer as glycerin significantly increases the flexibility of the film, but also reduces the tensile strength and elastic modulus [66].
Cellulose derivatives are more suitable for packaging that is in direct contact with food products [65]. Studies of the properties of various cellulose derivatives used for film formation are continuing with the aim of developing mixtures of cellulose derivatives with other biopolymers to improve the mechanical, barrier properties and increase the shelf life of packaged food products [46][67][68].
2.3. Pectin
Pectin is one of the main components of the plant cell wall, contributing to the integrity and rigidity of tissues, and is considered one of the most complex macromolecules in nature [69]. Although pectin is ubiquitous in the plant kingdom, pectin derived from apples, citrus fruits, sunflowers, and sugar beets is an undisputed commercial source for the processing industry due to their physical and chemical properties and the availability of biomass [70]. Pectin is a poly–α–1–4–galacturonic acid (Figure 4a) with different degrees of methylation of carboxylic acid residues and/or amidated polygalacturonic acids (Figure 4b) [71]. Carboxyl groups of galacturonic acid are esterificated with methanol, resulting in methoxylated carboxyl groups. On the other hand, amidated carboxyl groups are obtained when galacturonic acid is converted with ammonia to carboxylic acid amide.
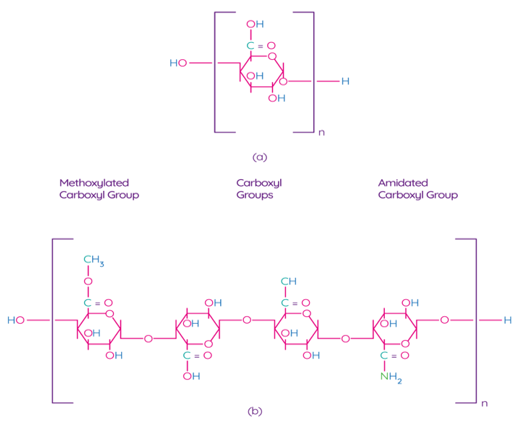
Figure 4. Chemical structure of polygalacturonic acid (a) and representative chemical structure of pectin, showing typical repeating groups (b) [72]. Reproduced with permission from Espitia, P.J.P. et al., Food Hydrocolloids; published by Elsevier Ltd., 2013.
According to the degree of esterification by methanol (the ratio of esterified galacturonic acid groups to its total number), pectin can be classified as high-methoxylpectin (HMP, > 50 % of esterified carboxyl groups) pectin or low-methoxyl pectin (LMP, < 50 % of esterified carboxyl groups) [70]. The degree of esterification affects the gelling properties of pectins. For example, pectin with a low content of methoxyl groups forms a gel in the presence of multivalent ions, which bonds pairs of carboxyl groups of different pectin chains. Pectin with a high content of methoxyl groups forms a gel in acidic solutions with the addition of various sugars, such as sucrose or glucose [72][73]. LMP is used for encapsulation, food packaging film processing, and low-calorie gels in dietary foods [72]. Structurally, pectins are classified as rhamnogalacturonan-I (RG-I; "hairy" pectin), substituted galacturonans (RG-II or SG; "hairy" pectin), and homogalacturonans (HG; smooth pectin). The hairy region of pectins (RG-I and RG-II) has a high probiotic potential [74]. Thus, based on the macromolecular and microstructural characteristics of pectins, the scope of their application in food products is determined.
Pectin is an ingredient used in the food industry without any restrictions other than current good manufacturing practices, is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA), and is used in food primarily as a gelling agent, stabilizing agent, or thickener in products such as jams, yogurt drinks, fruit milk drinks, and ice cream [75][76]. The ubiquitous presence, low cost, structural flexibility, and polymerization ability of pectin contribute to its use as a matrix for active food packaging materials [70]. Since bioactive packaging films made from pectin have very weak antimicrobial properties, their antimicrobial potential can be enhanced by integrating and combining them with various functional compounds, such as essential oils, phenolic compounds, nanomaterials, free fatty acids, and others [77]. The production of edible films from pectin can be carried out in various ways, such as casting, extrusion, spraying, and coating with a knife [78].
2.4. Chitosan
Chitosan is a linear polysaccharide consisting of randomly linked units of β-(1,4)-D-glucosamine and N-acetyl-D-glucosamine (Figure 5). Chitin is the second most common structural polysaccharide found in nature after cellulose and is usually deacetylated by an alkali for chitosan production [79][80]. Natively, chitin is presented in the form of ordered crystalline microfibrils that form structural components in the exoskeleton of arthropods or in the cell walls of fungi and yeast. So far, the main commercial sources of chitin are the shells of crabs and shrimps [81]. In industrial processing, chitin is extracted by acid treatment to dissolve calcium carbonate, and then by an alkaline solution to dissolve proteins. In addition, a discoloration stage is often added to remove the pigments and obtain a colorless pure chitin [82]. Chitin is usually isolated from the exoskeleton of crustaceans and, in particular, from shrimps and crabs, where α-chitin is produced [83]. Squid is another important source of chitin, in which it exists in the β-form, which has been found to be more amenable to deacetylation. Such chitin also shows higher solubility, reactivity, and affinity for solvents and swelling than α-chitin due to the much weaker intermolecular hydrogen bond attributed to the parallel arrangement of the main chains [83]. Some types of insects, fungi, bacteria, and algae can also be alternative sources of chitin/chitosan [84][85][86].
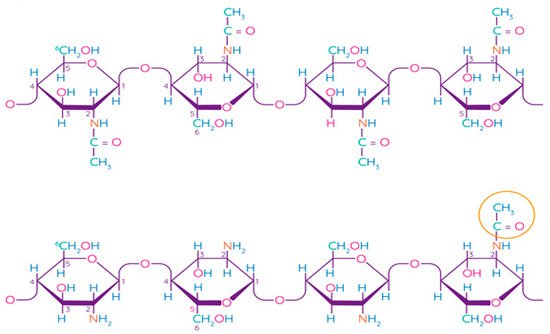
Figure 5. Chemical structure of chitin and chitosan [87]. Reproduced with permission from Rasul, R.M. et al., Carbohydrate Polymers; published by Elsevier Ltd., 2020.
The term “chitosan” usually refers to a family of polymers produced after the deacetylation of chitin to varying degrees [81]. In fact, the acetylation degree, which reflects the balance between the two types of residues, distinguishes chitin from chitosan. When the acetylation degree is higher 60%, the product is called chitosan and becomes soluble in acidic aqueous solutions [88]. A depolymerization reaction also occurs during deacetylation, as evidenced by changes in the molecular weight of chitosan. Chitin can be converted into chitosan by enzyme treatment [89] or by a chemical process [90]. Chemical methods are widely used for commercial purposes of producing chitosan due to their low cost and suitability for mass production [81]. Chitosan has been found to be non-toxic, biodegradable, bio-functional, and demonstrates good film-forming qualities and antimicrobial properties [91].
However, films prepared only from chitosan do not meet the criteria for packaging materials. They are rigid and brittle; therefore, it is important to use plasticizers to improve their mechanical properties. Various plasticizers have an effect on the stability of plasticized films. It was found that polyethylene glycol (PEG) and glycerin are the best candidates as plasticizers of chitosan films [92]. It was found that other small-molecular organic materials also improve the mechanical properties of chitosan films, but their effectiveness decreases within a few weeks [92]. The addition of PEG and glycerol promotes the formation of relatively flexible and stable (at least for several months) chitosan films. In general, these substances prevent the formation of double bonds between adjacent polymer chains, which prevents recrystallization [93]. Plasticizers modify the mechanical properties of chitosan without changing its fundamental chemical structure [92].
In comparison with other biopolymers used for food packaging, chitosan has an advantage due to its ability to include such functional substances as minerals or vitamins and its antibacterial activity, which is important for maintaining the quality of products [94][95][96].
2.5. Alginate
Alginates are natural indigestible polysaccharides, usually produced and purified from various genera of brown algae (mainly Laminaria hyperborean, Macrocystis pyrifera, Ascophyllum nodosum, and to a lesser extent, Laminaria digitate, Laminaria japonica, Eclonia maxima, Lesonia negrescens, Sargassum sp.) [97]. Some bacteria, such as Azotobacter vinelandii or mucoid strains of Pseudomonas aeruginosa, also synthesize alginate-like polymers as exopolysaccharides [98][99]. Azotobacter vinelandii is a strict aerobe that can fix nitrogen and synthesize two polymers during vegetative growth: alginate and intracellular polyesters (polyhydroxybutyrate) [100]. A. vinelandii produce alginate during the encystment process as a mechanism for increasing resistance to drying in adverse environmental conditions to maintain cell hydration, being its structural element [101]. Moreover, it can act as a protective barrier against heavy metal toxicity, creating an ion exchange system with selectivity for Ca2+ [102]. P. aeruginosa (mucoid phenotype) produce alginate as the causative agent of cystic fibrosis, and it is associated with pathogenicity. Alginate biosynthesis in Pseudomonas spp. is induced under dehydration conditions and is probably a key component of stable biofilms in various media [103].
The molecular structure of alginates consists of unbranched linear binary copolymers of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues bound by 1–4 glycosidic bonds (Figure 6) [97]. The structure of alginate algae can be divided into three fractions (three blocks of uronic acid): these are the homopolymer regions of the M and G blocks and the alternating MG blocks containing both polyuronic acids [104]. Bacterial alginates have O-acetyl groups, while in the structure of alginate algae, they are absent [105]. In addition, bacterial alginates have a higher molecular weight compared to algae polymers [106]. The source of alginate affects the ratio of M and G residues, which has an impact on the physical and chemical properties of alginate, as well as on solution viscosity and thickness of the film [97].
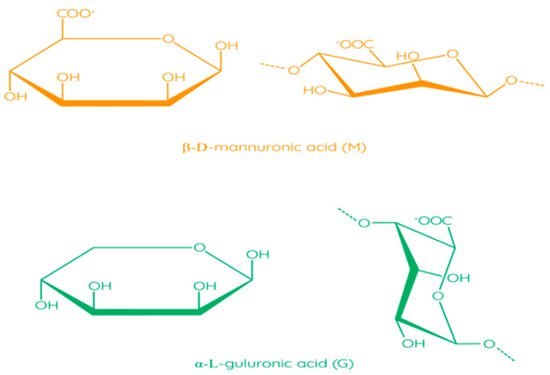
Figure 6. Structural formulas of monomers in alginate [97]. Reproduced from Parreidt, T.S. et al., Foods; published by MDPI, Basel, Switzerland, 2018.
The solubility of various types of alginates in numerous solvents and solutions was indicated by Kimica Corporation [107]. The FDA classifies food grade sodium alginate as a GRAS (generally considered safe) in the Code of Federal Regulations (CFP) and list its use as an emulsifier, stabilizer, thickener, and gelling agent [108]. The European Commission (EC) has added alginic acid and its salts (E400–E404) to the list of permitted food additives [109].
Alginate is widely used in various industries, such as food, beverage, textile, printing, and pharmaceutical, as a thickener, stabilizer, emulsifier, chelating agent, encapsulator, suspending agent, or used for the formation of gels, films, and membranes [110]. About 30,000 metric tons of alginate derived from brown algae are produced annually [100]. Alginate is known for its biocompatible and biodegradable properties, as well as its low price. The ability of functional groups of G blocks to interact with polyvalent cations (for example, Ca2+, Al3+, and Fe3+) is an important characteristic of alginate [111]. Among divalent ions, calcium ions usually react with alginate to form a polymer with low solubility. In general, the length of the G-block characterizes the ability and selectivity of the alginate in the formation of these interactions, whereas the M and MG blocks have almost no selectivity. The M and G blocks bind via ions to form a three-dimensional structure («egg-box») [112]. This triggers the process of anionic exchange, in which the water-soluble alginate exchanges its counter ions for Ca2+. This ionic crosslinking leads to the formation of cold-setting and heat-resistant films. Alginate films are cross-linked to improve their water resistance, mechanical properties, and coherence [111].
2.6. Casein
Caseins are suitable hydrocolloids for the formation of edible films among proteins due to their high nutritional value, solubility in water, and ability to emulsify. Casein is the main protein extracted from milk (~80%) [113], consisting of four different protein fractions: αS1-, αS2-, β-, and κ-casein [114], which together form colloidal micelles in milk (Figure 7) stabilized by casein structures and calcium-phosphate bridges [115]. The unique properties of the four protein fractions affect the film-forming ability of casein [116].
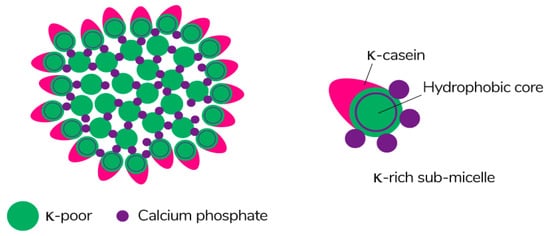
Figure 7. The schematic of the submicelle model of the casein micelle [117]. Reproduced with permission from Horne, D.S., Current Opinion in Colloid & Interface Science; published by Elsevier Ltd., 2005.
The global production of caseins and caseinates is difficult to determine due to the lack of significant data. The largest casein producers are New Zealand (150,000 tons), Netherlands (85,000–10,000 tons) and Germany (25,000–40,000 tons). The global market for casein or caseinates used in the food industry ranges from 200,000 to 2,500,000 tons [118].
Casein is precipitated from skimmed milk by acidifying it to produce acidic casein, or the milk is treated with rennet to produce rennet casein. The precipitated casein clot is separated from the serum, washed, and dried [119]. Caseinates are water-soluble derivatives of acidic caseins formed by reaction with alkalis. Edible casein is a dairy byproduct that is used as an ingredient in many foods, including dairy products [120]. The development of food technologies and their applications has increased the production of casein and the demand for it. Its production differs from that of non-food casein (also called industrial casein) because food casein is produced under sanitary conditions [118]. In addition, food-grade chemicals are used for its production that undergoes sufficient heat treatment to ensure that casein is safe for human consumption [118]. Intensive research on production technologies over the years and their implementation into factories has significantly improved the approaches for food-grade casein production.
Depending on the coagulation method, different types of casein with certain characteristics are obtained; rennet and acid casein are the main available types [121]. Acidic casein is insoluble in water, has a pH of about 4.6, and is precipitated by acidifying milk with mineral acids or lactic acid [113]. Rennet casein is insoluble in water, has a pH of about 7.5, and is coagulated by the action of chymosin, which cleaves the k-casein tail and causes destabilization of the casein micelles [114]. Acidic casein can be solubilized by neutralization with alkali, such as sodium, potassium, or calcium hydroxide, to produce sodium, potassium, or calcium caseinates, respectively. This caseinate does not contain colloidal calcium phosphates, has a pH close to 7, and is highly soluble in water [122].
Casein films have good barrier properties for oxygen and other non-polar molecules due to the distribution of polar amino acids along the protein chain, which allows them to protect products that are prone to oxidation [122]. On the other hand, the interaction forces between the polar and non-polar amino acids in the casein structure form a cohesive film matrix that tends to shrink during drying and becomes brittle [123]. Food-grade plasticizers, such as glycerin or sorbitol, are added to the film-forming solution in order to solve this problem. Plasticizers increase the thermoplasticity of the protein film but reduce its strength [124].
Although casein films have potential for use as food packaging, some disadvantages need to be addressed before they can be widely used for commercial purposes. Casein-based films are very sensitive to moisture, they absorb and release water molecules, which greatly affect their mechanical and barrier properties, and moreover, they are mostly soluble in water, which limits their areas of application [125]. It is also worth noting that plasticized casein films cannot provide high mechanical strength or good elasticity compared with synthetic polymer materials. By crosslinking polymer chains, chemical and physical processing can be used for modification of the polymer matrix, which improves the functionality of the protein film [126]. Glutaraldehyde or the natural compound genipin, as well as transglutaminase, etc. are typical chemicals used as crosslinking agents [114][127][128]. In general, aldehydes are quite effective as binding agents of protein molecules, but their high toxicity is unacceptable for foods. Genipin and transglutaminase are known to be safe, but their high cost limits their further use in industry [114]. Thus, the search for new cheap crosslinking agents safe for use in the food industry is underway.
2.7. Collagen
Collagen is the most abundant protein in the extracellular matrix of vertebrates, accounting for approximately 30% of the total body protein mass. It is absent in plants and unicellular organism, where its role is played by polysaccharides and cellulose. Collagen is presented in the body walls and cuticle in invertebrates, while in mammals, it is found in the cornea, bones, blood vessels, cartilage, dentin of teeth, etc. [129].
Bovine hides are a by-product of meat production and mainly used for the production of leather, but the inner corium layer of the hide is rich in collagen [130]. This collagen has a higher denaturation temperature compared to collagen from marine sources [131]. Collagen can also be extracted from fish and pigs, but there are some limitations: the use of fish collagen is limited because its low hydroxyproline content [132] gives collagen a low denaturation temperature, while pork collagen is restricted due to religious considerations [133].
Collagen consists of three parallel chains of α-helices twisted in the form of a right triple helix [129] and constructed from frequently repeated fragments with a characteristic sequence -Gly-X-Y- (Figure 8). Glycine is every third amino acid residue, proline is often found in the X position, and the Y position can be occupied by both proline and 4-hydroxyproline. In addition, the collagen molecule contains residues of 3-hydroxyproline and 5-hydroxylysine. The right triple helix self-associates to form highly ordered cross-linked fibrils [129]. These fibrils form insoluble fibers that provide an extracellular matrix with high integrity and mechanical tensile strength due to its tightly wound triple helical structure. Collagen molecules are divided into 26 different types, which are grouped into 8 families, depending on its structure, chain bonding, and position in the organism. These families include fibril-forming, basement membrane, anchoring fibrils, microfibrillar, hexagonal network-forming, transmembrane, multiplexins and fibril-associated collagens with interrupted triple helix [134].
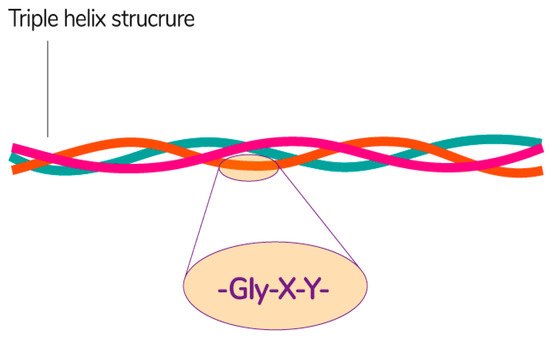
Figure 8. The general structural features of collagen [135]. Reproduced from Sebald, A., proof-read/edited by Mitchell, D.A. Maxfacts; published by University of York, 2019.
Water-soluble collagen presents in a small percentage of total collagen. The solubility of collagen depends on the type of tissue and age. A neutral salt solution or dilute acetic acid are the most commonly used solvents for collagen extraction. Strong alkali or enzymes are used to extract the insoluble collagen to break down the additional cross-linked bonds [136]. Collagen can be used for the production of edible films in the meat industry, e.g., for sausages, salami, snacks [136], due to its good mechanical properties [137]. Fibrillar collagens can easily form stable films capable of contracting and stretching to adapt to the compression and expansion of meat raw materials during continuous processing [138][139]. Collagen films can become an embedded/edible part of meat products so they can provide safety benefits, control quality changes, and reduce the loss of shrinkage of meat and meat-based products during storage, thereby extending the shelf life and maintaining the visual appeal of products for a long time [140].
2.8. Gelatin
Gelatin is a naturally occurring water-soluble protein characterized by the absence of a noticeable odor and the random configuration of polypeptide chains in an aqueous solution. It is obtained by partial hydrolysis of collagen [141]; its structure is rigid rod-shaped molecules that are located in fibers connected by covalent bonds [142].
Pig skin was used as a raw material for the production of gelatin in the 1930s and continues to be the most important material for the large-scale industrial production of food gelatin [143]. For more expensive applications, such as pharmaceuticals, gelatin is usually obtained from cattle bones, which is considered a more complex and expensive extraction process [144]. However, in an effort to replace pork and bovine gelatin, fish gelatin production has increased over the past decade, accounting for more than 1.5% of total gelatin production. In recent years, by-products obtained in the fishing industry, such as heads, skin, bones, fins, muscle parts, scales, internal organs, and others, are considered as potential sources of gelatin and not recyclable waste [145]. The main disadvantages of fish gelatin are its rheological properties, since it is less stable than mammalian gelatin. Moreover, since the production of gelatin from fish and poultry is still limited, the resulting products are less competitive in price than products made from mammalian gelatin [146][147].
Gelatin is a heterogeneous polypeptide mixture of α-chains, β-chains, and γ-chains (Figure 9).
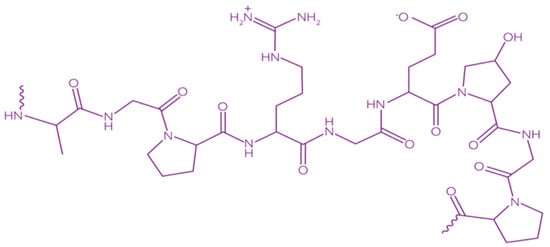
Figure 9. Chemical structure of gelatin [148]. Reproduced with permission from Kariduraganavar, M.Y., et al., Natural and Synthetic Biomedical Polymers; published by Elsevier Inc., 2014.
Gelatin can be divided into two types depending on the processing method. Type A has an isoelectric point at pH ~ 8–9 and is obtained from acid-treated collagen. Type B has an isoelectric point at pH ~ 4–5 and is obtained from an alkali-treated collagen, during which asparagine and glutamine residues are converted to their respective acids, resulting in a higher viscosity [149]. Gelatin obtained from pig skin is commonly referred to as type A, and gelatin obtained from beef skin or cattle skins and bones is referred to as type B.
Gelatin has various functional properties that can be divided into two groups: properties related to the external surface, such as protective colloidal function, formation and stabilization of emulsion and foam, adhesion and cohesion, as well as the ability to form films and properties related to gelation, such as thickening, texturing, and water-binding ability [150][151][152]. Thus, a wide range of gelatin applications can be used in the food, packaging, pharmaceutical, cosmetic, and photographic industries, but the limited thermal stability and mechanical properties of gelatin, especially during processing, limit its implementation [139].
The film-forming properties of gelatin are widely used as an outer film to protect food products from drying out, exposure to light and/or oxygen during their shelf life [153]. Due to the high hygroscopicity of gelatin, it tends to swell or dissolve upon contact with the surface of foods with high moisture content [154].
Several scientific studies have been conducted to evaluate the overall effect of adding crosslinking and strengthening agents, plasticizers, or additives with antimicrobial or antioxidant properties to gelatin-based films to improve its functional properties and increase the shelf life of food products [155][156]. The improvement of these properties occurs when the intermolecular interactions of the protein chains decrease under the influence of molecular structures that modify their hydrophilic character or promote the formation of strong covalent bonds in the protein film [157].
Zhao et al. demonstrated the feasibility of using natural extracts as new natural crosslinking agents for the modification of gelatin (type B, from bovine bone) by forming hydrogen bonds between water and free hydroxyl groups of amino or polyphenolic groups, which significantly increased the strength of the gel compared to untreated gelatin [158]. Combining gelatin with other biopolymers, such as whey proteins, starch, chitosan, or pectin, can also be a good way to improve the mechanical properties and water resistance of gelatin-based films [159][160][161][162].
This entry is adapted from the peer-reviewed paper 10.3390/polym13101592
References
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51.
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Chapter 1 Sources of Chitin and Chitosan and their Isolation. In Chitin and Chitosan: Properties and Applications, 1st ed.; van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2019; pp. 1–34.
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367.
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69.
- Majtán, J.; Bíliková, K.; Markovič, O.; Gróf, J.; Kogan, G.; Šimúth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241.
- Yen, M.-T.; Mau, J.-L. Physico-chemical characterization of fungal chitosan from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 472–479.
- Rasul, R.M.; Muniandy, M.T.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800.
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614.
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181.
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the Plasticizing Effects of Glycerol and PEG 400 on Chitosan Films Using Solid-State NMR Spectroscopy. Macromolecules 2009, 42, 4667–4673.
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597.
- Bilbao-Sainz, C.; Chiou, B.-S.; Williams, T.; Wood, D.; Du, W.-X.; Sedej, I.; Ban, Z.; Rodov, V.; Poverenov, E.; Vinokur, Y.; et al. Vitamin D-fortified chitosan films from mushroom waste. Carbohydr. Polym. 2017, 167, 97–104.
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888.
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67.
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170.
- Emmerichs, N.; Wingender, J.; Flemming, H.-C.; Mayer, C. Interaction between alginates and manganese cations: Identification of preferred cation binding sites. Int. J. Biol. Macromol. 2004, 34, 73–79.
- Evans, L.R.; Linker, A. Production and Characterization of the Slime Polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 1973, 116, 915–924.
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650.
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial alginate production: An overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 2017, 33, 198.
- Flores, C.; Díaz-Barrera, A.; Martínez, F.; Galindo, E.; Peña, C. Role of oxygen in the polymerization and de-polymerization of alginate produced by Azotobacter vinelandii. J. Chem. Technol. Biotechnol. 2015, 90, 356–365.
- Hay, I.D.; Wang, Y.; Moradali, M.F.; Rehman, Z.U.; Rehm, B.H.A. Genetics and regulation of bacterial alginate production. Environ. Microbiol. 2014, 16, 2997–3011.
- Draget, K.I. Chapter 29 Alginates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 807–828.
- Davidson, I.W.; Sutherland, I.W.; Lawson, C.J. Localization of O-Acetyl Groups of Bacterial Alginate. J. Gen. Microbiol. 1977, 98, 603–606.
- Clementi, F. Alginate Production by Azotobacter Vinelandii. Crit. Rev. Biotechnol. 1997, 17, 327–361.
- Kimica Corporation. How to Use Alginates. Available online: (accessed on 11 May 2020).
- U.S. Food & Drug Administration. Code for Federal Regulations Title 21 Part 184—Direct Food Substances Affirmed as Generally Recognized as Safe. Available online: (accessed on 11 May 2020).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA J. 2017, 15.
- Kim, Y.-J.; Yoon, K.-J.; Ko, S.-W. Preparation and properties of alginate superabsorbent filament fibers crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804.
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers 2019, 11, 1594.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389.
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039.
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434.
- Bonnaillie, L.M.; Zhang, H.; Akkurt, S.; Yam, K.L.; Tomasula, P.M. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018–2036.
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18 - Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 477–500.
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153.
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 676–682.
- Lawrence, N.D.; Kentish, S.E.; O’Connor, A.J.; Barber, A.R.; Stevens, G.W. Microfiltration of skim milk using polymeric membranes for casein concentrate manufacture. Sep. Purif. Technol. 2008, 60, 237–244.
- Alekseev, G.V.; Khripov, A.A. Method of Rapid Remote Control of Casein Concentration in Dairy Products in Unopened Packages. J. Food Process Eng. 2015, 38, 11–18.
- Carr, A.; Golding, M. Functional Milk Proteins Production and Utilization: Casein-Based Ingredients. In Advanced Dairy Chemistry, 4th ed.; McSweeney, P., O’Mahony, J., Eds.; Springer: New York, NY, USA, 2016; Volume 1B, pp. 35–66.
- Chevalier, E.; Assezat, G.; Prochazka, F.; Oulahal, N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll. 2018, 75, 182–191.
- Xu, J.-L.; Gowen, A.A. Investigation of plasticizer aggregation problem in casein based biopolymer using chemical imaging. Talanta 2019, 193, 128–138.
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58.
- Ucpinar Durmaz, B.; Aytac, A. Poly (vinyl alcohol) and casein films: The effects of glycerol amount on the properties of films. Res. Eng. Struct. Mater. 2019, 5, 155–165.
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584.
- Lin, H.-C.; Wang, B.-J.; Weng, Y.-M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT 2020, 118, 108813.
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; Di Pierro, P.; Mariniello, L.; Porta, R. Chapter 21—Transglutaminase Cross-Linked Edible Films and Coatings for Food Applications. In Enzymes in Food Biotechnology, 1st ed.; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–388.
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 123–127.
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55.
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961.
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38.
- Goyal, D.; Goyal, A.; Brittberg, M. Consideration of religious sentiments while selecting a biological product for knee arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1577–1586.
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26.
- Sebald, A. Collagen. 2019. Available online: (accessed on 25 February 2021).
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42.
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351.
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227.
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41.
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168.
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198.
- Kaewruang, P.; Benjakul, S.; Prodpran, T. Molecular and functional properties of gelatin from the skin of unicorn leatherjacket as affected by extracting temperatures. Food Chem. 2013, 138, 1431–1437.
- Mariod, A.A.; Fadol, H.A. Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147.
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92.
- Siburian, W.Z.; Rochima, E.; Andriani, Y.; Praseptiangga, D. Fish gelatin (definition, manufacture, analysis of quality characteristics, and application): A review. Int. J. Fish. Aquat. Stud. 2020, 8, 90–95.
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1—Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31.
- Lee, B.; Lum, N.; Seow, L.; Lim, P.; Tan, L. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797.
- Aksun Tümerkan, E.T.; Cansu, Ü.; Boran, G.; Mac Regenstein, J.; Özoğul, F. Physiochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279.
- Chandra, M.V.; Shamasundar, B.A. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584.
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M.; Barkia, A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 309–318.
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495.
- Scopel, B.S.; Ribeiro, M.E.; Dettmer, A.; Baldasso, C. Cornstarch-Gelatin Films: Commercial Gelatin Versus Chromed Leather Waste Gelatin and Evaluation of Drying Conditions. J. Polym. Environ. 2018, 26, 1998–2006.
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci 2016, 133.
- Ortiz-Zarama, M.A.; Jiménez-Aparicio, A.R.; Solorza-Feria, J. Obtainment and partial characterization of biodegradable gelatin films with tannic acid, bentonite and glycerol. J. Sci. Food Agric. 2016, 96, 3424–3431.
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119.
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of Gelatine with Galla chinensis Extract, a Natural Crosslinker. Int. J. Food Prop. 2016, 19, 731–744.
- Alemán, A.; González, F.; Arancibia, M.Y.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Comparative study between film and coating packaging based on shrimp concentrate obtained from marine industrial waste for fish sausage preservation. Food Control 2016, 70, 325–332.
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319.
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64.
- Taylor, M.M.; Lee, J.; Bumanlag, L.P.; Latona, R.J.; Brown, E.M. Biopolymers produced from gelatin and whey protein concentrate using polyphenols. J. Am. Leather Chem. Assoc. 2014, 109, 82–88.
