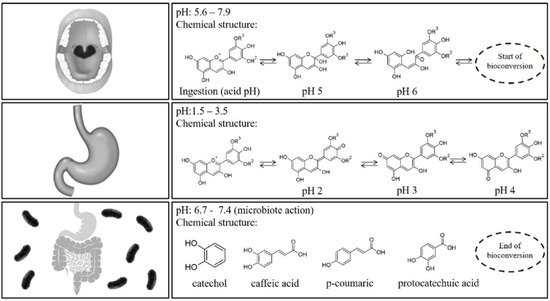
The daily intake of anthocyanins can be estimated via food databases and can range from few to hundreds of milligrams per person due to the methodological differences in the assessment, together with the influence of nutritional, cultural, and social differences of the investigated populations [
8]. The pattern followed by anthocyanins after oral dispensation is unique and different from other flavonoids [
77]. Anthocyanins have a markedly low bioavailability, only 1–2% of the ingested anthocyanins maintain their parent C6–C3–C6 structure in the organism. Food digestion is a pH-dependent process and, therefore, anthocyanins are subjected to transformations in addition to hydrolyzation by several enzymes in the small intestine [
12,
78]. A portion of the ingested anthocyanins reaches the large intestine, where they are metabolized into low-molecular-weight catabolites, which can be excreted in the feces within 2–4 h (up to 8 h) or absorbed again. Active transporters through either gastric or intestinal cell barrier play an important role in their transfer and absorption within the liver, kidney, brain, or other organs and tissues, besides the stomach [
13,
79]. In a recent review on tissue bioavailability in animals, Sandoval-Ramírez et al. [
80] concluded that the TAC absorbed was 2.17 × 10
5 pmol/g in mice kidney, 1.73 × 10
5 pmol/g in liver, 3.6 × 10
3 pmol/g in heart, and 1.16 × 10
5 pmol/g in lung; and 6.08 × 10
3 pmol/g in pig brain. In the wall of the intestine and then in the liver, anthocyanins and their catabolites undergo phase 2 enzymatic metabolism being also transformed into their glucuronidated, sulphated, and methylated forms [
10,
12,
13,
14,
78,
81,
82,
83]. The presence of microbial catabolites at many sites of the body, at higher concentration than the native form, has suggested that part of the biological activities attributed to anthocyanins is related to the synergetic effect of their colonic catabolites [
13,
84]. Anthocyanin metabolites and transformation products have been characterized and quantified by several authors [
85,
86,
87,
88,
89]. Ferrars et al. [
88] identified a wide variety of anthocyanin phenolic metabolites, including 11 novel metabolites, in post-menopausal women after 12 weeks elderberry intake, at concentration levels higher than their anthocyanin native forms. There are many critical factors affecting the fate of anthocyanins and their metabolites in our organism: the ability to cross membranes, pH, digestive enzymes, microbiota, biliary acids, or food matrix. The use of radiolabeled (
14C) or stable isotope–labelled (
13C) tracer studies provides useful information about in which extent anthocyanins are metabolized to phenolic acid derivatives. In this sense, Czank et al. [
90] investigated the fate of anthocyanins in eight male participants after the ingestion of
13C-cyanidin-3-
O-glucoside (500 mg). The relative mean bioavailability was 12.38% (5.37% excreted in urine and 6.91% in breath). The authors found maximum serum concentration 42-fold higher for
13C-labeled metabolites than their respective native compound
13C-cyanidin-3-glucoside. Up to 49 metabolites were detected including among others: phase II conjugates of cyanidin-3-glucoside and cyanidin (cyanidin-glucuronide, methyl cyanidin-glucuronide, and methyl cyanidin-3-glucoside-glucuronide); degradation products (protocatechuic acid, phloroglucinaldehyde, and phloroglucinaldehyde); phase II conjugates of protocatechuic acid, phenylacetic acids, phenylpropenoic acids, and hippuric acid.
The mechanisms through which anthocyanins may exert their bioactivity are not fully understood as it is not clear whether their activity is linked to native forms, their derivatives, or both. The distinction of their different biological roles is a very challenging task. Some comparative studies have been conducted on the antioxidant activity of anthocyanin metabolites [
91]. Recently, Kim et al. [
92] provided basic information of the chemical changes of cyanidin glycosides during in vitro gastrointestinal digestion. Cyanidin-3-
O-galactoside was degraded into caffeoylquinic acid, which was not found after in vitro digestion of cyanidin-3-
O-glucoside. The bioactivity (DPPH) of the anthocyanin metabolites decreased in the intestinal fraction. However, the bioactivity increased after simulated colonic digestion, possibly because of the newly formed colonic metabolites. Furthermore, anthocyanin metabolites from the chokeberry extract exhibited higher DPPH radical activities than those from the mulberry extract. In another study, α-glucosidase inhibitory activity and ROS scavenging activities of conjugated-pelargonidin-3-
O-glucoside samples were potentially increased after gastrointestinal digestion [
93].
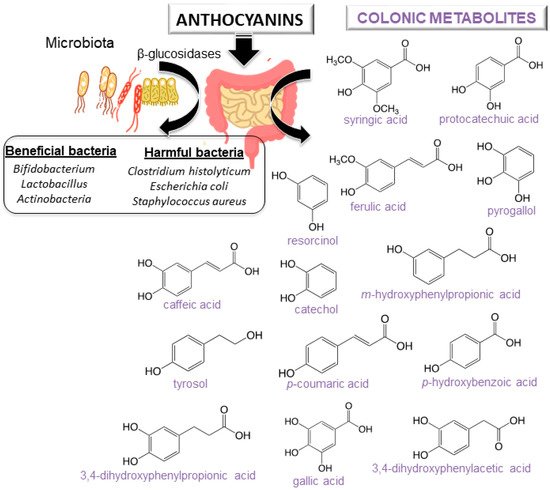
For a better understanding of the anthocyanin bioavailability, different in vivo and in vitro models simulating digestion have been proposed [
11]. Gowd et al. [
94] assessed the phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation at different time intervals (0–48 h). Authors revealed the formation of gut metabolites enhance the high glucose plus palmitic acid induced ROS, mitochondrial membrane collapse, and glutathione depletion in HepG2 cells. Several studies have also reported that after anthocyanin colonic fermentation occurs an increase of beneficial bacteria (
Bifidobacterium spp.,
Actinobacteria,
Bacteroidetes,
Lactobacillus/Enterococcus spp.,
Akkermansia) [
95,
96,
97,
98,
99,
100]. Intestinal microbiota possesses β-glucosidase activity, allowing the release of glucose from the aglycone and providing energy to support bacterial growth. A study recently carried out by Zhou et al. [
95] suggests that the consumption of blueberry and its extracts could exert prebiotic activity and a modulatory effect on the composition and abundance of human intestinal microbiota. Anthocyanins could enhance human health by modulating gut microorganisms, which are often related to different diseases [
95,
101]. Nevertheless, it is important to note that anthocyanin derivatives can also reduce some harmful bacteria such as
C. histolyticum after colonic fermentation [
101,
102]. A summary of anthocyanins colonic metabolism metabolites and colon microbiota alteration is shown in [
11].
3. Therapeutic Effects of Anthocyanins
Available scientific studies prove the beneficial effects of the presence of anthocyanins in fruits and vegetables in the prevention of diseases [
60,
66,
81,
112]. Even after the ingestion of high doses of anthocyanin and derivatives no negative effects have been observed [
113]. This section covers the main health benefits of anthocyanins in different types of pathologies including eye health, cardiovascular disease, antiobesity, antidiabetic, antimicrobial effects, anticancer activities, and neurodegenerative disorders. A summary of the positive effects of anthocyanins is shown in [
114,
115,
116,
117,
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132,
133,
134,
135,
136,
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147,
148,
149,
150,
151,
152,
153,
154,
155,
156,
157,
158,
159,
160], and their mechanisms of action in disease prevention are discussed below.
Table 3. Health benefits of anthocyanins.
| Eye Health |
Administration |
References |
| Improvement of vision in patients with open-angle glaucoma |
Oral capsule |
[114] |
| Protective effect during retinal inflammation |
IV in rats |
[115] |
| Regeneration of rhodopsin and smooth muscle relaxation |
IV in mouse model |
[116] |
| Improvement of dark adaptation |
Oral capsule |
[117] |
| Prevention of cataractogenesis of diabetic cataract |
Incubation of Enucleated rat lenses |
[118] |
| Antiapoptotic effects against oxidative damage of lens epithelial cell |
Cell studies |
[119] |
| Prevention of retinal degeneration induced by N-methyl-N-nitrosourea |
Oral solution |
[120] |
| Increase of ocular blood flows |
Oral capsule |
[121] |
| Cardiovascular diseases |
|
|
| Inhibition of platelet aggregation (in vitro antithrombotic properties) |
Cell studies |
[122] |
| Increase of high-density lipoprotein cholesterol levels and decrease of low-density lipoprotein cholesterol levels |
Oral capsule |
[123] |
| Lower risk of non-fatal myocardial infarction |
Oral intake |
[124] |
| Vasorelaxation properties in isolated coronary artery rings in pigs |
Cell studies |
[125] |
| Decrease of susceptibility to ischemia-reperfusion injury and infarct size |
Rodent food |
[126] |
| Improvement of lipid profile and platelet function |
Oral capsule |
[127] |
| Antiobesity effects |
|
|
| Improvement of weight gain and lipid profile on obese rats |
Fat diet-induced mouse model |
[128] |
| Suppression of body weight gain and improve blood lipid profile in rats |
Fat diet-induced mouse model |
[129] |
| Reduction of sugar concentration in urine and plasma in rats |
Intraperitoneal and intragastric administration |
[130] |
| Ameliorated obesity in high-fat-fed mice |
Cell studies |
[131] |
| Upregulation of adipocytokine secretion and gene expression in rat adipocytes |
Cell studies |
[132] |
| Suppression of fat tissue gain, weight gain and other metabolic disorders |
Fat diet-induced mouse model |
[133] |
| Antidiabetic effects |
|
|
| Amelioration of hyperglycemia and insulin sensitivity in diabetic mice |
Fat diet-induced mouse model |
[134] |
| Improvement of dyslipidemia, enhancement of antioxidant capacity, and prevention of insulin resistance in human with type 2 diabetes |
Oral capsule |
[135] |
| Alleviation of glomerular angiogenesis of diabetic kidneys in mice |
Cell studies |
[136] |
| Inhibition of DPP IV activity (a protease that regulates blood glucose levels via degradation of incretins) |
Computational studies |
[137] |
| Amelioration of renal apoptosis in diabetic nephropathy mice |
Oral solution |
[138] |
| Activation of adipose tissue-derived adiponectin to defend against diabetes-related endothelial dysfunction in mice |
Diet-induced mouse model |
[139] |
| Antimicrobial effects |
|
|
| Induction of cell damage by destroying the cell wall, membrane, and intercellular matrix |
Cell studies |
[140] |
| Highest sensitivity to Aeromonas hydrophila and Listeria innocua |
Microbial strains |
[141] |
| Antibacterial effects towards Enterococcus faecium resistant to vancomycin, Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli |
Microbial strains |
[142] |
| Inhibition of Gram-negative bacteria |
Microbial strains |
[143] |
| Anticancer effects |
|
|
| Suppression of cell proliferation, inflammation, and angiogenesis and induction of apoptosis in esophageal tissue of rats |
Diet-induced rat model |
[144] |
| Anti-invasive potential in breast cancer cell lines |
Cell studies |
[145] |
| Anticancer effect on BALB/c nude mice bearing MDA-MB-453 cell xenografts and breast cancer cell lines |
Cell studies |
[146] |
| Inhibition of cell migration and invasion, suppression of activation of rapidly accelerated fibrosarcoma, mitogen-activated protein kinase and c-Jun N-terminal kinase, and downregulation of secretion of matrix metalloproteinase 2 |
Cell studies |
[147] |
| Inhibition of growth of human HT-29 colon cancer cells, increase of expression of tumor suppression genes and decrease of cyclooxygenase-2 gene expression |
Cell studies |
[148] |
| Reduction of colonic aberrant crypt foci, colonic cellular proliferation and COX-2 mRNA expression in rats |
Diet-induced rat model |
[149] |
| Suppression of formation of aberrant crypt foci in colons of CF-1 mice |
Cell studies and diet-induced rat model |
[150] |
| Promotion of apoptosis in benign prostatic hyperplasia rats |
Oral doses in rat model |
[151] |
| Anti-invasive effect on human hepatoma Hep3B cells and inhibition of matrix metalloproteinase MMP-2 and MMP-9 gene expression |
Cell studies |
[152] |
| Inhibition of Akt-mTOR signaling thereby inducing maturation of acute myeloid leukemia cells, besides inducing apoptotic players such as TRAIL in cancer systems |
Cell studies |
[153] |
| Neurodegenerative diseases |
|
|
| Neuroprotective activity by suppression of dopaminergic cell death in Parkinson’s disease |
Cell studies |
[154] |
| Improvement of learning and memory ability in mice. Higher antioxidant enzyme activity and less lipid oxidation in both brain and liver |
Diet-induced mouse model |
[155] |
| Regulation of cholinergic neurotransmission to restore Na+, K+-ATPase and Ca2+-ATPase activities and to prevent memory deficits in rats |
Oral and injected rat models |
[156] |
| Neuroprotective effect: Memory and synaptic dysfunction |
Oral rat models |
[157] |
| Improvement of its free radical scavenging capabilities via p38/JNK pathway against Abeta1-42-induced oxidative stress |
Cell studies |
[158] |
| Enhancement of neuroprotection against Abeta1-42-induced neuroinflammation and neurodegeneration |
Oral mouse model and cell studies |
[159] |
| Enhancement of the neuroprotection in an Abeta1-42 mouse model of Alzheimer’s disease |
Oral mouse model and cell studies |
[160] |
Eye health: Since the first report in 1966 about the positive effects of anthocyanins on vision in humans, anthocyanin-rich extracts have been worldwide utilized as a popular supplement for ocular health [
161,
162]. Oral dispensation of blackcurrant anthocyanins may be a promising supplement for patients with open-angle glaucoma, being also effective for antiglaucoma medication, while anthocyanin-rich bilberry extract has a protective effect on vision during retinal inflammation [
115]. It has also been confirmed that cyanidin helps the regeneration of rhodopsin and smooth muscle relaxation in rats [
116]. Results have also showed that bilberry extracts were able to suppress the photoxidation of pyridinium disretinoid A2E, an auto-fluorescence pigment that accumulates in retinal epithelial cells with age and can cause light-induced damage to the cell. In a comparative study a significant improvement on nocturnal visual function and an improved contrast sensitivity levels in subjects with myopia versus placebo group was observed [
114]. Anthocyanins act also inhibiting transient myopia, reducing eye fatigue or enhancing retinal blood flow with glaucoma [
118,
121,
161,
163].
Cardiovascular diseases: It is especially important the role of anthocyanins in preventing myocardial infarction and cardiovascular disease related to mortality. Extracts of anthocyanins have been used to inhibit platelet aggregation being preventive in the initial stage of thrombi; in the treatment of problem with poor micro-circulation resulting from capillary fragility; and also to prevent the LDL oxidation [
122,
164,
165,
166]. In a placebo-controlled trial in dyslipidemia patients (40–65 years) the intake of berry-derived anthocyanins improved lipoprotein profile through cholesteryl ester transfer protein inhibition [
123]. Authors observed a greater increase in high-density lipoprotein (HDL) cholesterol levels and in the cellular cholesterol efflux to serum as well as a decrease in LDL cholesterol levels in the anthocyanin group in contrast to the placebo group. Similar results were reported by Álvarez Suárez et al. [
127] in an in vivo study using healthy volunteers supplemented with strawberries (500 g). Daily consumption improved the lipid profile reducing total cholesterol, LDL cholesterol and triglycerides levels, while HDL cholesterol remained unchanged. This increased antihemolytic defenses and platelet function in the subjects. In another attempt, higher intakes of fruit-based anthocyanins were associated to a lower risk of nonfatal myocardial infarction (14%) and ischemic stroke in a prospective cohort study in men over 24 years [
124]. A meta-analysis of 45 randomized controlled trials stated that the consumption of berries and purified anthocyanins (2.2−1230 mg anthocyanins/day) increases significantly HDL-cholesterol and reduces LDL-cholesterol, triglycerides, systolic blood pressure, and diastolic blood pressure as well as the inflammatory markers CRP and TNFα [
167]. The analysis also suggested that some individuals are more susceptible to the protective effects of anthocyanin consumption: (i) overweight; (ii) over 50 years; and (iii) those with increased risk of cardiovascular disease. Another meta-analysis of 99 randomized controlled trials showed that the consumption of anthocyanin rich-products decreased significantly both systolic and diastolic blood pressure regardless of the health status of the participants [
168].
In in vitro assays, anthocyanins have also shown inhibition of the porcine pancreatic elastase [
169], an enzyme that plays a significant function in pathologies such as arteriosclerosis, emphysema, or rheumatoid arthritis, etc., by attacking fibers and collagen. Moreover, acceleration in the cicatrization process due to anthocyanin-rich extract has been demonstrated, showing preventive and curative activity against gastroduodenal ulcers induced in rats [
7]. Their influence on the biosynthesis of mucopolysaccharides provably improves the efficacy of the gastric mucous layer, and increases the base substance of the connective tissue and of the capillaries [
170].
Antiobesity and Antidiabetic effects: Anthocyanins have shown anti-obesity effects through multiple mechanisms such as inhibiting lipid absorption, regulating lipid metabolism, increasing energy expenditure, suppressing food intake and regulating gut microbiota, which suggests anthocyanins are promising candidates in anti-obesity therapies [
171]. Kwon et al. [
128] observed that anthocyanins-added diet from black soybean in rats decreases body weight gains, being significantly lowered in the rats fed with a high fat diet plus black soybean anthocyanins compared with the rats fed with high fat diet without black soybean. Anthocyanins also improved the lipid profile and suppressed the high fat diet-induced weight gain in liver intermediately and decreased the weights of epididymal and perirenal fat pads.
In addition, type 2 diabetes is closely related to obesity [
66]. Anthocyanins can alleviate complications in type 2 diabetes by inhibiting intestinal glucose absorption, inducing pancreatic insulin secretion, upregulating glucose transporter type 4, and suppressing hepatic gluconeogenesis [
172]. After the supplementation of a high-fat diet during 13 weeks with different berries in mice, Heyman et al. [
173] observed that those supplemented mice gained lesser body weight and presented lower fasting insulin levels than the control group as well as mediated positive effects on glucose homeostasis. Jankowski et al. [
130] described a substantial decrease in the sugar concentration in urine and blood serum after streptozotocin injection in fed rats with grapes. The mechanisms of anthocyanins suggested by the authors were the reduction of the biosynthesis of collagen, lipoproteins, and glycoproteins, as well as the reduction of the activity of elastase and adenosine deaminase (both high in diabetic patients). Treatment with cherries in rats resulted in a significant reduction of blood glucose and urinary microalbumin and an increase of the creatinine secretion level in urea [
174]. The pulp, seed and skin from “red chilto” (a red fruit from Argentina) had a hypoglycemic effect and acted increasing glucose absorption, decreasing glucose diffusion rate and promoting glucose transport across the cell membrane [
175] in an in vitro simulated gastroduodenal digestion. Consumption of blueberries and apples/pears in humans was also associated to a lower risk of type 2 diabetes [
176].
Antimicrobial effects: The antimicrobial activity of anthocyanins against a wide range of microorganisms is also well documented. Possible mechanisms induced cell damage by destroying the cell wall, membrane and intercellular matrix [
66,
140,
177]. Blackberry extracts have antibacterial activity with the highest sensitivity to
Aeromonas hydrophilia and
Listeria innocua [
141]. Cranberry extracts have antibacterial activity towards
Enterococcus faecium resistant to vancomycin,
Pseudomonas aeruginosa,
Staphylococcus aureus, and
Escherichia coli [
142]. Different types of berry extracts inhibit Gram-negative bacteria but not Gram-positive bacteria [
143] probably because Gram-negative bacteria acts as a preventive barrier against hydrophobic compounds but not against hydrophilic compounds [
178].
Anticancer activity: Possible mechanisms of the anticancer activity of anthocyanins have been described by many authors: antimutagenic activity; inhibition of oxidative DNA damage and carcinogen activation; induction of phase II enzymes for detoxification; cell cycle arrest; inhibition of cyclooxygenase-2 enzymes; as well as induction of apoptosis and antiangiogenesis [
179,
180,
181,
182,
183,
184].
In breast cancer, anthocyanins cause the inhibition of key modulators that promote its progression and development by acting directly in the DNA fragmentation and promoting the death of MCF-7 cancer cells [
185,
186]. In addition, the studies indicate that anthocyanins exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. For example, delphinidin can act as a potential antimetastatic agent that suppresses PMA-induced cancer cell invasion through the specific inhibition of NF-κB-dependent MMP-9 gene expression [
187,
188]. In lung cancer, the treatment of cyanidin-3-glucoside and cyanidin 3-rutinoside, isolated from mulberry, inhibits the migration and invasion of A549 cells and also decreases MMP-2 and uPA and enhances TIMP-2 and PAI. Anthocyanins also inhibit the growth of carcinogenic cells that provoke colon cancer, induce the apoptosis effect, and are even able to act as modulators of the macrophages in the immune response [
180]. Forester et al. [
189] also reported the positive effect of anthocyanin metabolites decreasing cell viability and causing cell cycle arrest and apoptosis in colon cancer. In oral and cervical cancer, the invasion of SCC-4 cells and HeLa cells were diminished by the treatment of peonidin 3-glucoside and cyanidin-3-glucoside [
190].
It is also important to note that the structures of anthocyanins have a considerable influence on their biological activities [
191,
192,
193]. In this sense, the type of aglycones, sugars, and acylated acids, and the position and degree of glycosylation and acylation seem to be the main factors influencing the anticancer property [
191]. Jing et al. [
192] compared the anticancer properties of anthocyanin-rich extracts using human colon cancer HT29 cell line. Authors reported the following growth inhibitory activity rates: purple corn > chokeberry and bilberry > purple carrot and grape > radish and elderberry. Those non-acylated monoglycosylated anthocyanins had greater anticancer property than those with pelargonidin, triglycoside, and/or acylation with cinnamic acid.
Neurodegenerative diseases: Anthocyanins are also uniquely suited for the treatment of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, or amyotrophic lateral sclerosis. Their main mechanisms include antioxidant pathways, calcium homeostasis, inflammation, protein homeostasis, and the balance of pro-survival and pro-apoptotic signaling [
194,
195].
In a primary cell model of Parkinson’s disease, dopaminergic cell death elicited by rotenone was suppressed by extracts prepared from blueberries, grape seed, hibiscus, blackcurrant, and mulberry [
154]. Moreover, Strathearn et al. [
154] observed that those extracts rich in anthocyanins and proanthocyanidins exhibited greater neuroprotective activity than extracts rich in other polyphenols.
The oral dispensation of anthocyanins (200 mg/kg) in rats was able to regulate cholinergic neurotransmission, to restore Na
+, K
+-ATPase and Ca
2+-ATPase activities, and to prevent memory deficits caused by scopolamine dispensation [
156]. Rehman et al. [
157] showed the neuroprotective effect of anthocyanins based on an artificial ageing model using D-galactose to induce oxidative stress and inflammatory response. The potential mechanisms of their action included: decreased expression of the receptor for advance glycation end product, reduced level of ROS, and lipid peroxidation. Shih et al. [
155] observed that mice fed with anthocyanin-rich mulberry extracts demonstrated significantly less amyloid β protein and showed improvement of learning and memory ability in avoidance response tests. The fed mice also showed a higher antioxidant enzyme activity and less lipid oxidation in both brain and liver, as compared to the control mice. Besides, the treatment with anthocyanin-rich mulberry extract has been proved to decrease the levels of serum aspartate aminotransferase, alanine aminotransferase, triglyceride, and total cholesterol that increase with ageing.
Furthermore, the therapeutic profile of anthocyanins can be improved by encapsulation [
158,
159,
160]. For instance, in Alzheimer’s disease Amin et al. [
158] showed that encapsulated nanoparticles loaded with anthocyanins are rapidly taken up by cells enhancing their neuroprotective profile against amyloid beta toxicity above that of anthocyanins alone. Similar activity was also observed in in vivo studies in mice [
158,
160].