The intervertebral disc (IVD) is a complex joint structure comprising three primary components—namely, nucleus pulposus (NP), annulus fibrosus (AF), and cartilaginous endplate (CEP). The IVD retrieves oxygen from the surrounding vertebral body through CEP by diffusion and likely generates ATP via anaerobic glycolysis. IVD degeneration is characterized by a cascade of cellular, compositional, structural changes. With advanced age, pronounced changes occur in the composition of the disc extracellular matrix (ECM). NP and AF cells in the IVD possess poor regenerative capacity compared with that of other tissues. Hypoxia-inducible factor (HIF) is a master transcription factor that initiates a coordinated cellular cascade in response to a low oxygen tension environment, including the regulation of numerous enzymes in response to hypoxia. HIF-1α is essential for NP development and homeostasis and is involved in various processes of IVD degeneration process, promotes ECM in NP, maintains the metabolic activities of NP, and regulates dystrophic mineralization of NP, as well as angiogenesis, autophagy, and apoptosis during IVD degeneration. HIF-1α may, therefore, represent a diagnostic tool for early IVD degeneration and a therapeutic target for inhibiting IVD degeneration
- hypoxia-inducible factor-1α
- regeneration
- intervertebral disc
1. IVD and HIF
2. Structure and Function of the IVD
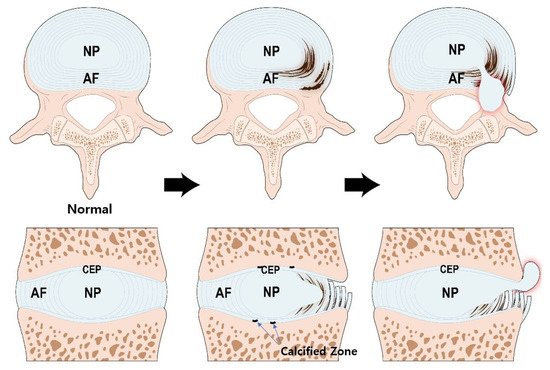
3. Pathogenesis of IVD Degeneration
4. Expression of HIF and Signal Transduction of HIF-1α in IVD
4.1. Expression Patterns of HIF-1α and HIF-2α in IVD
4.2. Signal Transduction Pathway of HIF-1α in IVD
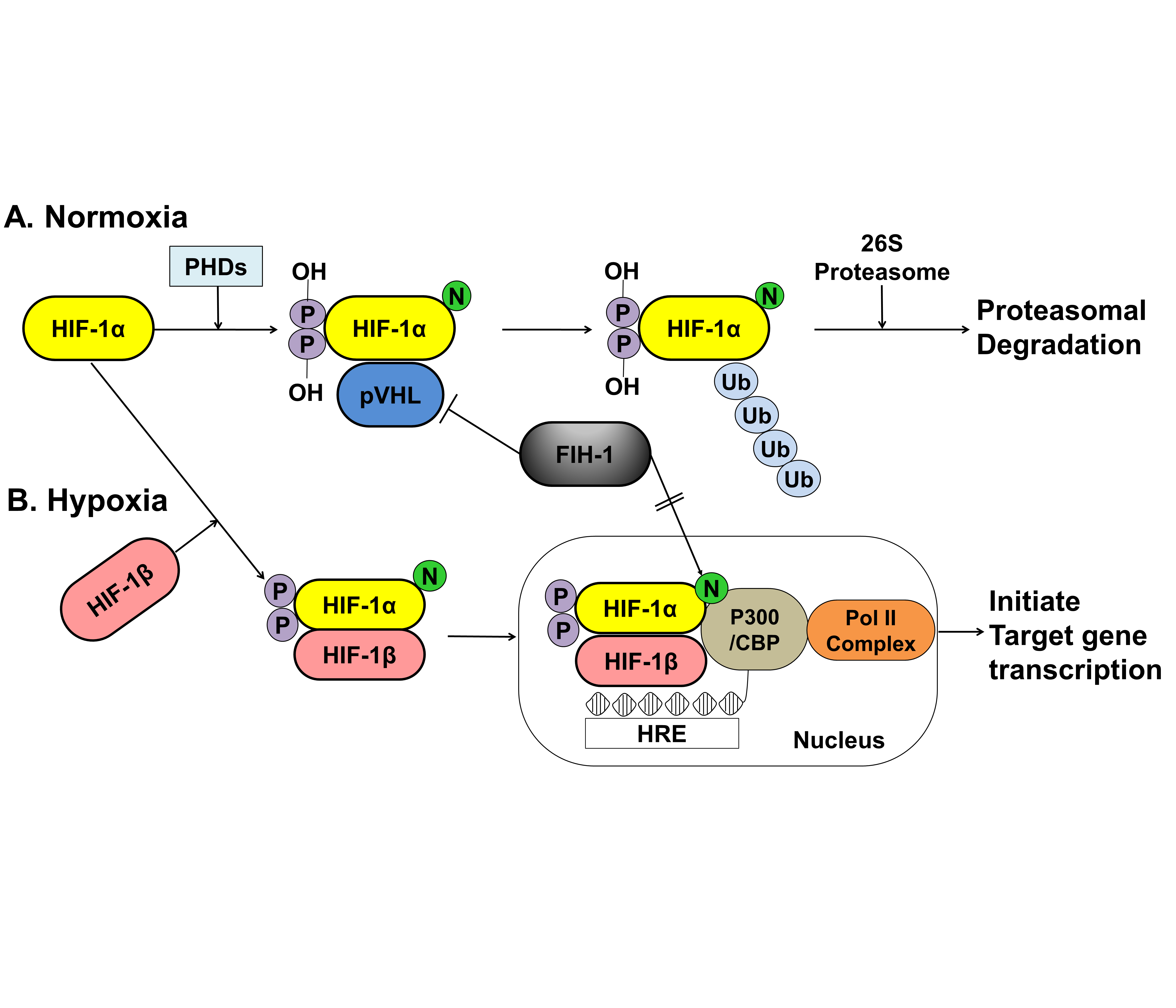
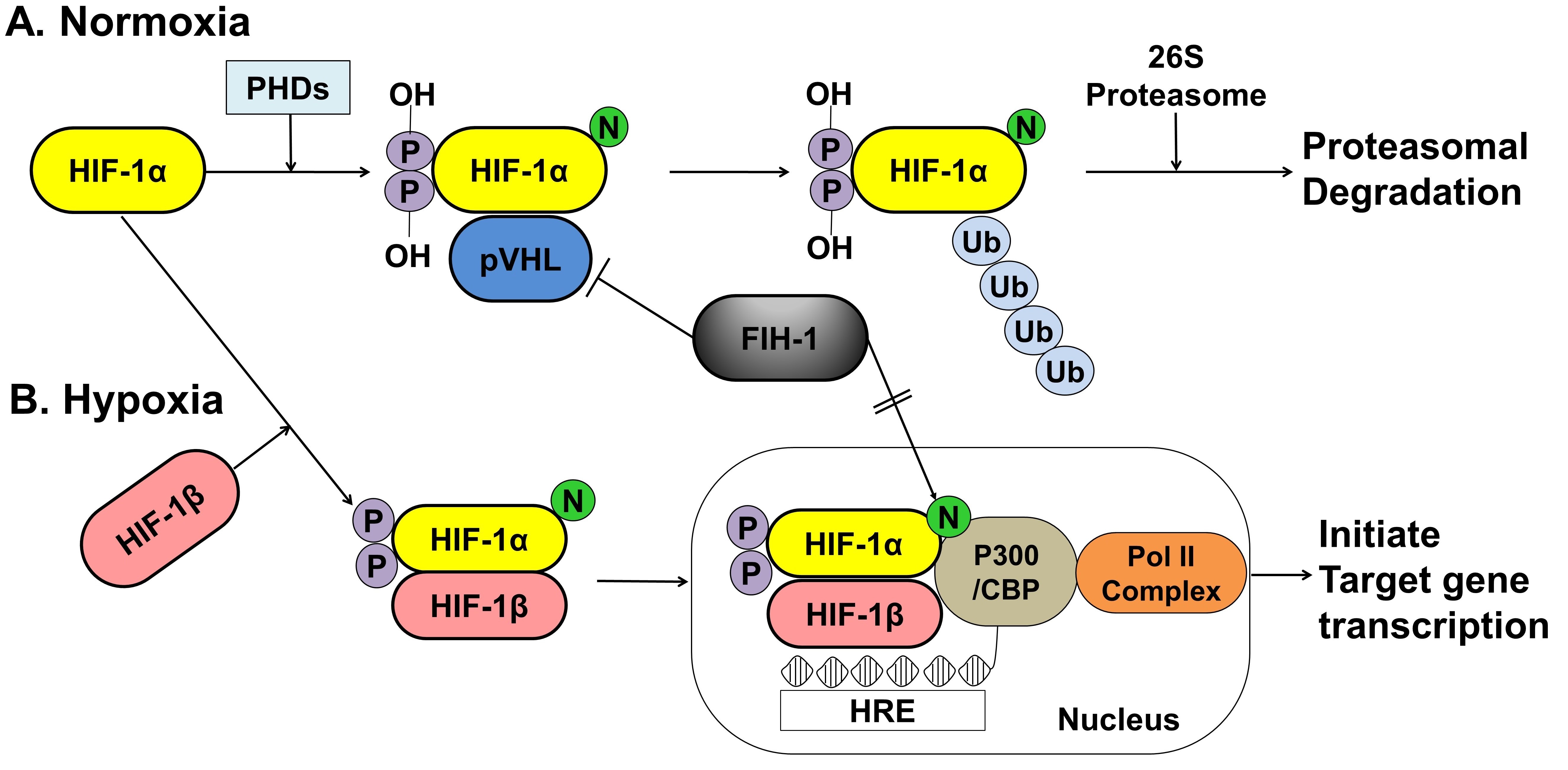
Figure 2. A schematic representation of the oxygen-dependent regulation of HIF-1α under normoxic and hypoxic conditions. (A) Under normoxic conditions, HIF-1α is hydroxylated at specific proline residues by PHD enzymes on the proline residues, which leads to polyubiquitination and proteasomal degradation of HIF-1α by the VHL protein, a component of E3 ubiquitin-protein ligase. (B) Under hypoxic conditions, PHD enzyme activity is inhibited, thereby allowing HIF-1α to accumulate and translocate to the nucleus, where it dimerizes with HIF-1β and binds to the HRE sequences of target gene promoters. The FIH-1, an asparagine hydroxylase, regulates the transcriptional activity of HIF-1 binding to VHL and represses HIF-1 transactivation by preventing the binding of the transcriptional coactivator p300/CBP to the HIF-1α. HIF; hypoxia inducible factor, PHDs; prolyl hydroxylase domain proteins, pVHL; protein von Hippel Lindau, CBP; Creb-binding protein, HRE; hypoxia-response element, Pol II; DNA polymerase II complex, Ub; Ubiquitous, FIH-1; factor inhibiting HIF-1.
5. Regeneration for IVD Degeneration—Focused on HIF-1α
5.1. Main Roles of HIF-1α in IVD Degeneration
5.1.1. Promotion of Extracellular Matrix in NP Cells
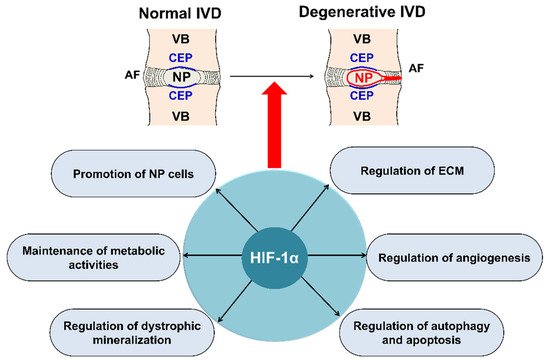
5.1.2. Maintenance of the Metabolic Activity of NP Cells
5.1.3. Regulation of Dystrophic Mineralization in NP Cells
5.1.4. Regulation of Angiogenesis during IVD Degeneration
5.1.5. Autophagy and Apoptosis during IVD Degeneration
5.2. HIF-1α Development Strategies for IVD Regeneration
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms22105281
References
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battie, M.C.; Seguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123.
- Choi, H.; Tessier, S.; Silagi, E.S.; Kyada, R.; Yousefi, F.; Pleshko, N.; Shapiro, I.M.; Risbud, M.V. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 2018, 70, 102–122.
- Tian, Y.; Yuan, W.; Li, J.; Wang, H.; Hunt, M.G.; Liu, C.; Shapiro, I.M.; Risbud, M.V. TGFβ regulates Galectin-3 expression through canonical Smad3 signaling pathway in nucleus pulposus cells: Implications in intervertebral disc degeneration. Matrix Biol. 2016, 50, 39–52.
- Korecki, C.L.; MacLean, J.J.; Iatridis, J.C. Dynamic compression effects on intervertebral disc mechanics and biology. Spine 2008, 33, 1403–1409.
- Berg-Johansen, B.; Fields, A.J.; Liebenberg, E.C.; Li, A.; Lotz, J.C. Structure-function relationships at the human spinal disc-vertebra interface. J. Orthop. Res. 2018, 36, 192–201.
- Fields, A.J.; Ballatori, A.; Liebenberg, E.C.; Lotz, J.C. Contribution of the endplates to disc degeneration. Curr. Mol. Biol. Rep. 2018, 4, 151–160.
- Bartels, E.M.; Fairbank, J.C.; Winlove, C.P.; Urban, J.P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 1998, 23, 1–7; discussion 8.
- Taylor, J.R. Growth of human intervertebral discs and vertebral bodies. J. Anat. 1975, 120, 49–68.
- Fujita, N.; Markova, D.; Anderson, D.G.; Chiba, K.; Toyama, Y.; Shapiro, I.M.; Risbud, M.V. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: Distinct roles of PHD2 and PHD3 proteins in controlling HIF-1alpha activity in hypoxia. J. Biol. Chem. 2012, 287, 16975–16986.
- Rudert, M.; Tillmann, B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop. Scand. 1993, 64, 37–40.
- Weber, K.T.; Jacobsen, T.D.; Maidhof, R.; Virojanapa, J.; Overby, C.; Bloom, O.; Quraishi, S.; Levine, M.; Chahine, N.O. Developments in intervertebral disc disease research: Pathophysiology, mechanobiology, and therapeutics. Curr. Rev. Musculoskelet. Med. 2015, 8, 18–31.
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655.
- Li, Y.; Liu, S.; Pan, D.; Xu, B.; Xing, X.; Zhou, H.; Zhang, B.; Zhou, S.; Ning, G.; Feng, S. The potential role and trend of HIF-1α in intervertebral disc degeneration: Friend or foe? (Review). Mol. Med. Rep. 2021, 23.
- Li, H.; Liang, C.Z.; Chen, Q.X. Regulatory role of hypoxia inducible factor in the biological behavior of nucleus pulposus cells. Yonsei Med. J. 2013, 54, 807–812.
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709.
- Urban, J.P. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30 Pt 6, 858–864.
- Richardson, S.M.; Knowles, R.; Tyler, J.; Mobasheri, A.; Hoyland, J.A. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. HistoChem. Cell Biol. 2008, 129, 503–511.
- Semenza, G.L. Hypoxia-inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 2001, 7, 345–350.
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490.
- Zhao, J.; Zhang, P.; Qin, L.; Pan, X.H. Hypoxia is essential for bone-tendon junction healing: The molecular biological evidence. Int. Orthop. 2011, 35, 925–928.
- Agrawal, A.; Gajghate, S.; Smith, H.; Anderson, D.G.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008, 58, 3798–3808.
- Podjarny, E.; Bernheim, J.; Hasdan, G.; Karsh, D.; Rashid, G.; Green, J.; Katz, B.; Bernheim, J. Additive renoprotective effect of candesartan and tetrahydrobiopterin in rats after 5/6 nephrectomy. Nephrol. Dial. Transplant. 2007, 22, 1864–1872.
- Risbud, M.V.; Guttapalli, A.; Stokes, D.G.; Hawkins, D.; Danielson, K.G.; Schaer, T.P.; Albert, T.J.; Shapiro, I.M. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J. Cell Biochem. 2006, 98, 152–159.
- Rajpurohit, R.; Risbud, M.V.; Ducheyne, P.; Vresilovic, E.J.; Shapiro, I.M. Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002, 308, 401–407.
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480.
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454.
- Fujita, N.; Chiba, K.; Shapiro, I.M.; Risbud, M.V. HIF-1alpha and HIF-2alpha degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 2012, 27, 401–412.
- Gogate, S.S.; Nasser, R.; Shapiro, I.M.; Risbud, M.V. Hypoxic regulation of β-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: Role of hypoxia-inducible factor proteins. Arthritis Rheum. 2011, 63, 1950–1960.
- Skubutyte, R.; Markova, D.; Freeman, T.A.; Anderson, D.G.; Dion, A.S.; Williams, C.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia-inducible factor regulation of ANK expression in nucleus pulposus cells: Possible implications in controlling dystrophic mineralization in the intervertebral disc. Arthritis Rheum. 2010, 62, 2707–2715.
- Zeng, Y.; Danielson, K.G.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J. Bone Miner. Res. 2007, 22, 1851–1861.
- Agrawal, A.; Guttapalli, A.; Narayan, S.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am. J. Physiol. Cell Physiol. 2007, 293, C621–C631.
- Ha, K.Y.; Koh, I.J.; Kirpalani, P.A.; Kim, Y.Y.; Cho, Y.K.; Khang, G.S.; Han, C.W. The expression of hypoxia inducible factor-1alpha and apoptosis in herniated discs. Spine 2006, 31, 1309–1313.
- Neucere, J.N.; Godshall, M.A. Effects of base-soluble proteins and methanol-soluble polysaccharides from corn on mycelial growth of Aspergillus flavus. Mycopathologia 1991, 113, 103–108.
- Merceron, C.; Mangiavini, L.; Robling, A.; Wilson, T.L.; Giaccia, A.J.; Shapiro, I.M.; Schipani, E.; Risbud, M.V. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS ONE 2014, 9, e110768.
- Meng, X.; Zhuang, L.; Wang, J.; Liu, Z.; Wang, Y.; Xiao, D.; Zhang, X. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol. 2018, 11, 548–557.
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314.
- Adams, P.; Eyre, D.R.; Muir, H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol. Rehabil. 1977, 16, 22–29.
- Walker, M.H.; Anderson, D.G. Molecular basis of intervertebral disc degeneration. Spine J. 2004, 4 (Suppl. S6), 158S–166S.
- Jordao, H.W.; McKenna, G.; McMenamin, U.C.; Kunzmann, A.T.; Murray, L.J.; Coleman, H.G. The association between self-reported poor oral health and gastrointestinal cancer risk in the UK Biobank: A large prospective cohort study. United Eur. Gastroenterol. J. 2019, 7, 1241–1249.
- Feng, H.; Danfelter, M.; Stromqvist, B.; Heinegard, D. Extracellular matrix in disc degeneration. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 25–29.
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410.
- Humzah, M.D.; Soames, R.W. Human intervertebral disc: Structure and function. Anat. Rec. 1988, 220, 337–356.
- Roughley, P.J.; Melching, L.I.; Heathfield, T.F.; Pearce, R.H.; Mort, J.S. The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 2006, 15 (Suppl. S3), S326–S332.
- Hukins, D.W. A simple model for the function of proteoglycans and collagen in the response to compression of the intervertebral disc. Proc. Biol. Sci. 1992, 249, 281–285.
- Wade, K.R.; Robertson, P.A.; Broom, N.D. A fresh look at the nucleus-endplate region: New evidence for significant structural integration. Eur. Spine J. 2011, 20, 1225–1232.
- Wu, Y.; Cisewski, S.E.; Wegner, N.; Zhao, S.; Pellegrini, V.D., Jr.; Slate, E.H.; Yao, H. Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J. Biomech. 2016, 49, 2756–2762.
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130.
- Nachemson, A.; Lewin, T.; Maroudas, A.; Freeman, M.A. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop. Scand. 1970, 41, 589–607.
- Ogata, K.; Whiteside, L.A. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine 1981, 6, 211–216.
- Grunhagen, T.; Wilde, G.; Soukane, D.M.; Shirazi-Adl, S.A.; Urban, J.P. Nutrient supply and intervertebral disc metabolism. J Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 30–35.
- Ashinsky, B.G.; Gullbrand, S.E.; Wang, C.; Bonnevie, E.D.; Han, L.; Mauck, R.L.; Smith, H.E. Degeneration alters structure-function relationships at multiple length-scales and across interfaces in human intervertebral discs. J. Anat. 2021, 238, 986–998.
- Holm, S.; Maroudas, A.; Urban, J.P.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect Tissue Res. 1981, 8, 101–119.
- Bibby, S.R.; Jones, D.A.; Ripley, R.M.; Urban, J.P. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 2005, 30, 487–496.
- Ishihara, H.; Urban, J.P. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop. Res. 1999, 17, 829–835.
- Bibby, S.R.; Urban, J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004, 13, 695–701.
- Ohshima, H.; Urban, J.P. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine 1992, 17, 1079–1082.
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349.
- Bernick, S.; Cailliet, R. Vertebral end-plate changes with aging of human vertebrae. Spine 1982, 7, 97–102.
- Grant, M.P.; Epure, L.M.; Bokhari, R.; Roughley, P.; Antoniou, J.; Mwale, F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc. Eur. Cell Mater. 2016, 32, 137–151.
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002, 27, 2631–2644.
- Garfin, S.R.; Eismont, F.J.; Bell, G.R.; Bono, C.M.; Fischgrund, J.S. The Intervertebral Disc: Normal, Aging, and Pathologic. In Rothman-Simeone and Herkowitz’s the Spine, 7th ed.; Olsen, S.A., Kang, J.D., Vo, N.V., Sowa, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 79–89.
- Eyre, D.R.; Muir, H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976, 157, 267–270.
- Duance, V.C.; Crean, J.K.; Sims, T.J.; Avery, N.; Smith, S.; Menage, J.; Eisenstein, S.M.; Roberts, S. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine 1998, 23, 2545–2551.
- Madhu, V.; Boneski, P.K.; Silagi, E.; Qiu, Y.; Kurland, I.; Guntur, A.R.; Shapiro, I.M.; Risbud, M.V. Hypoxic Regulation of Mitochondrial Metabolism and Mitophagy in Nucleus Pulposus Cells Is Dependent on HIF-1α-BNIP3 Axis. J. Bone Miner. Res. 2020, 35, 1504–1524.
- Huang, Y.; Wang, Y.; Wu, C.; Tian, W. Elevated expression of hypoxia-inducible factor-2α regulated catabolic factors during intervertebral disc degeneration. Life Sci. 2019, 232, 116565.
- Buckley, D.L.; Van Molle, I.; Gareiss, P.C.; Tae, H.S.; Michel, J.; Noblin, D.J.; Jorgensen, W.L.; Ciulli, A.; Crews, C.M. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J. Am. Chem. Soc. 2012, 134, 4465–4468.
- Liu, Y.; Lei, Y.; Guo, S.; Zuo, Z. Ensemble-based virtual screening in discovering potent inhibitors targeting Von Hippel-Lindau (VHL) E3 ubiquitin ligase. Life Sci. 2020, 262, 118495.
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686.
- Chen, T.; Ren, Z.; Ye, L.C.; Zhou, P.H.; Xu, J.M.; Shi, Q.; Yao, L.Q.; Zhong, Y.S. Factor inhibiting HIF1alpha (FIH-1) functions as a tumor suppressor in human colorectal cancer by repressing HIF1alpha pathway. Cancer Biol. Ther. 2015, 16, 244–252.
- Feng, X.; Yu, X.; Pang, M.; Tong, J. Molecular characterization and expression regulation of the factor-inhibiting HIF-1 (FIH-1) gene under hypoxic stress in bighead carp (Aristichthys nobilis). Fish. Physiol. Biochem. 2019, 45, 657–665.
- Park, J.B.; Chang, H.; Kim, K.W. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine 2001, 26, 618–621.
- Zhu, G.B.; Jiang, X.R.; Xia, C.L.; Sun, Y.J.; Zeng, Q.S.; Wu, X.M.; Li, X.C. Association of FAS and FAS ligand polymorphisms with the susceptibility and severity of lumbar disc degeneration in Chinese Han population. Biomarkers 2011, 16, 485–490.
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80.
- Vasiliadis, E.S.; Pneumaticos, S.G.; Evangelopoulos, D.S.; Papavassiliou, A.G. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol. Med. 2014, 20, 400–409.
- Wang, S.Z.; Chang, Q.; Lu, J.; Wang, C. Growth factors and platelet-rich plasma: Promising biological strategies for early intervertebral disc degeneration. Int. Orthop. 2015, 39, 927–934.
- Chen, S.; Fang, X.Q.; Wang, Q.; Wang, S.W.; Hu, Z.J.; Zhou, Z.J.; Xu, W.B.; Wang, J.Y.; Qin, A.; Fan, S.W. PHD/HIF-1 upregulates CA12 to protect against degenerative disc disease: A human sample, in vitro and ex vivo study. Lab. Investig. 2016, 96, 561–569.
- Feng, G.; Li, L.; Hong, Y.; Liu, H.; Song, Y.; Pei, F.; Ma, P.X.; Gong, Q.; Gupte, M.J. Hypoxia promotes nucleus pulposus phenotype in 3D scaffolds in vitro and in vivo: Laboratory investigation. J. Neurosurg. Spine 2014, 21, 303–309.
- Feng, G.; Li, L.; Liu, H.; Song, Y.; Huang, F.; Tu, C.; Shen, B.; Gong, Q.; Li, T.; Liu, L.; et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthr. Cartil. 2013, 21, 582–588.
- Wang, X.; Lv, G.; Li, J.; Wang, B.; Zhang, Q.; Lu, C. LncRNA-RP11-296A18.3/miR-138/HIF1A Pathway Regulates the Proliferation ECM Synthesis of Human Nucleus Pulposus Cells (HNPCs). J. Cell Biochem. 2017, 118, 4862–4871.
- Hiyama, A.; Skubutyte, R.; Markova, D.; Anderson, D.G.; Yadla, S.; Sakai, D.; Mochida, J.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011, 63, 1355–1364.
- Liu, Z.; Li, C.; Meng, X.; Bai, Y.; Qi, J.; Wang, J.; Zhou, Q.; Zhang, W.; Zhang, X. Hypoxia-inducible factor-lalpha mediates aggrecan and collagen Pi expression via NOTCH1 signaling in nucleus pulposus cells during intervertebral disc degeneration. Biochem. Biophys. Res. Commun. 2017, 488, 554–561.
- Tannenbaum, A.; Silverstone, H. Failure to inhibit the formation of mammary carcinoma in mice by intermittent fasting. Cancer Res. 1950, 10, 577–579.
- Benita, Y.; Kikuchi, H.; Smith, A.D.; Zhang, M.Q.; Chung, D.C.; Xavier, R.J. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009, 37, 4587–4602.
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197.
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417.
- Kirsch, T. Determinants of pathological mineralization. Curr. Opin. Rheumatol. 2006, 18, 174–180.
- Shao, J.; Yu, M.; Jiang, L.; Wei, F.; Wu, F.; Liu, Z.; Liu, X. Differences in calcification and osteogenic potential of herniated discs according to the severity of degeneration based on Pfirrmann grade: A cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 191.
- Guiot, B.H.; Fessler, R.G. Molecular biology of degenerative disc disease. Neurosurgery 2000, 47, 1034–1040.
- Pendleton, A.; Johnson, M.D.; Hughes, A.; Gurley, K.A.; Ho, A.M.; Doherty, M.; Dixey, J.; Gillet, P.; Loeuille, D.; McGrath, R.; et al. Mutations in ANKH cause chondrocalcinosis. Am. J. Hum. Genet. 2002, 71, 933–940.
- Williams, C.J.; Pendleton, A.; Bonavita, G.; Reginato, A.J.; Hughes, A.E.; Peariso, S.; Doherty, M.; McCarty, D.J.; Ryan, L.M. Mutations in the amino terminus of ANKH in two US families with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2003, 48, 2627–2631.
- Liu, M.H.; Sun, C.; Yao, Y.; Fan, X.; Liu, H.; Cui, Y.H.; Bian, X.W.; Huang, B.; Zhou, Y. Matrix stiffness promotes cartilage endplate chondrocyte calcification in disc degeneration via miR-20a targeting ANKH expression. Sci. Rep. 2016, 6, 25401.
- Takahashi, S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol. Pharm. Bull. 2011, 34, 1785–1788.
- Han, I.B.; Ropper, A.E.; Teng, Y.D.; Shin, D.A.; Jeon, Y.J.; Park, H.M.; Shin, D.E.; Park, Y.S.; Kim, K.N.; Kim, N.K. Association between VEGF and eNOS gene polymorphisms and lumbar disc degeneration in a young Korean population. Genet. Mol. Res. 2013, 12, 2294–2305.
- Haro, H.; Kato, T.; Komori, H.; Osada, M.; Shinomiya, K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J. Orthop. Res. 2002, 20, 409–415.
- Doita, M.; Kanatani, T.; Ozaki, T.; Matsui, N.; Kurosaka, M.; Yoshiya, S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001, 26, 1522–1527.
- Kwon, W.K.; Moon, H.J.; Kwon, T.H.; Park, Y.K.; Kim, J.H. The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells During Inflammatory Reactions. Neurosurgery 2017, 81, 867–875.
- Wu, W.J.; Zhang, X.K.; Zheng, X.F.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int. J. Immunopathol. Pharmacol. 2013, 26, 601–609.
- Mazure, N.M.; Pouysségur, J. Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell Biol. 2010, 22, 177–180.
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38.
- Tu, J.; Li, W.; Li, S.; Liu, W.; Zhang, Y.; Wu, X.; Luo, R.; Hua, W.; Wang, K.; Song, Y.; et al. Sestrin-Mediated Inhibition of Stress-Induced Intervertebral Disc Degradation Through the Enhancement of Autophagy. Cell Physiol. Biochem. 2018, 45, 1940–1954.
- Chen, J.W.; Ni, B.B.; Zheng, X.F.; Li, B.; Jiang, S.D.; Jiang, L.S. Hypoxia facilitates the survival of nucleus pulposus cells in serum deprivation by down-regulating excessive autophagy through restricting ROS generation. Int. J. Biochem. Cell Biol. 2015, 59, 1–10.
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785.
- Gruber, H.E.; Hanley, E.N., Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 1998, 23, 751–757.
- Zhou, X.; Li, J.; Teng, J.; Liu, Y.; Zhang, D.; Liu, L.; Zhang, W. microRNA-155-3p attenuates intervertebral disc degeneration via inhibition of KDM3A and HIF1α. Inflamm. Res. 2021, 70, 297–308.
- Wang, W.; Deng, G.; Qiu, Y.; Huang, X.; Xi, Y.; Yu, J.; Yang, X.; Ye, X. Transplantation of allogenic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int. J. Mol. Med. 2018, 41, 2553–2564.
- Huang, Z.; Zhang, L.; Feng, X.; Chen, T.; Bi, S. A new in vivo method to retard progression of intervertebral disc degeneration through stimulation of endogenous stem cells with simvastatin. Med. Hypotheses 2017, 101, 65–66.
- Guo, W.; Zhang, B.; Mu, K.; Feng, S.Q.; Dong, Z.Y.; Ning, G.Z.; Li, H.R.; Liu, S.; Zhao, L.; Li, Y.; et al. Circular RNA GRB10 as a competitive endogenous RNA regulating nucleus pulposus cells death in degenerative intervertebral disk. Cell Death Dis. 2018, 9, 319.
