The phytochemical constituents present in conifer extracts are nontoxic at therapeutic levels, with polyphenolic compounds having significant biological activities. Stilbenes, terpenes, alkaloids, lignins and flavanoids, such as quercetin, rutin, resveratrol, and the compounds PYC and enzogenol, are the phytochemical components of conifer extracts reported to have sedative, antidiabetic, anticancer and anesthetic effects. In addition, phytochemicals present in conifer extracts assist in the regulation of glucose and lipid metabolism, insulin secretion, stimulating β cells, the NF-kB signaling pathway, the inhibition of gluconeogenic enzymes, ROS protective action as well as targeting and modulating cytokines which affect neuron cells and reduce oxidative stress.
- conifers
- phytoconstituent
- biological effects
- phytomedicine
1. Background
| Nitrogen Compounds | |||
| Alkaloids | Vellosimine, 1,6-dehydropinidine, cis-pinidine, 1,6-dehydropinidinone, epipinidinone, cis-pinidinol, trans-pinidine, euphococcinine, α-pipecoline 1, (−)-pinidine | P. mariana, P. abies, P. sabiniana, P. torreyana, | [13,18,28] |
| Lignans | Lariciıesinol, taxiresinol, 3’-demethylisolariciresino1-9’-hydroxyisopropylethe, isolariciresinol, deoxypodophyllotoxin, (−)-secoisolariciresinol, 3, 3-demethylisolariciresinol, isotaxiresinol 2, α-conidendrin, (+)-pinoresinol, (−)-matairesinol, arctiin, dibenzylbutyrolactol, (−)-wikstromol, (−)-traxillagenin, (−)-arctigenin, traxillaside, 4′-deme-thyltraxillagenin, [(2R,3R)-2-(4′’-hydroxy-3′’-methoxybenzyl)-3-(4′-hydroxy-3′,5′dimethoxybenzyl)-butyrolactone] | T. baccata, J. taxifolia, J. sabina, J. virginiana, J. virginiana, P. roxburghii, Cedrus deodara, T. nucifera | [29,30,31,32,33,34,35] |
| Polyphenols: Flavonoids | |||
| Flavanonols | Taxifolin, cedeodarin | C. deodara, L. simbraca, P. roxburghii, P. mariana, P. abies, A. pindrow, A. excelsa; P. pinea, P. halepensis, P. pinaster, P. gerardiana | [33,36,37,38,39,40] |
| Flavones | Pilosanol B, luteolin, apigenin, apigenin 6-C-b-glucopyranoside | P. mariana, A. excelsa, P. abies, P. sylvestris, P. menziesii, P. menziesii, J. communis, A. angustifolia, L. deciduas | [18,38,39,41,42,43] |
| Biflavones | Bilobetin, cupressuflavone II-7-O-methyl-robustaflavone | T. wallichiana, C. macrocarpa, A. angustifolia | [43,44,45,46] |
| Flavonols | Quercetin, dihydroquercetin, rutin, kaempferol, dihydrokaempferol | J. communis, J. oxycedrus, P. gerardiana, P. roxburghii, P. wallichiana, A. angustifolia, P. abies, L. deciduas, P. sylvestris, P. menziesii, M. glyptostroboides, J. excelsa, P. mariana, J. foetidissima | [18,33,41,42,43,47,48,49,50] |
| Flavan-3-ols | Monomers: (−)-epicatechin, (−)-epicatechin-3-gallate, (+)-catechin, sennidin A, (−)-epigallocatechin, | P. pinaster, P. pinea, P. halepensis, P. roxburghii, P. wallichiana, P. gerardiana, J. foetidissima, A. angustifolia, P. abies, L. deciduas, P. sylvestris, J. communis, P. menziesii, J. oxycedrus, M. glyptostroboides, J. excelsa | [18,33,39,40,41,43,47,48,49,50] |
| Polymers: Procyanidin B1, B2, procyanidin A2, | P. halepensis, P. pinea, P. pinaster | [40] | |
| Phenolic acids | |||
| Benzoic acids | p-hydroxybenzoic acid, 2,5-dihydroxobenzoic acid, gallic acid, 4-hydroxybenzoic acid, protocatechuic acid, ellagic acid | P. abies, L. deciduas, P. sylvestris, P. menziesii, P. kesiya, J. communis, A. excelsa, P. roxburghii, P. wallichiana, P. gerardiana, L. deciduas, J. communis | [33,38,41] |
| Hydroxycinnamic acid | Caffeic acid, t-cinnamic Acid, p-coumaric acid, vanillic acid, ferulic acid, salicylic acid, sinapic acid, syringic acid, chlorogenic acid, 5-caffeoylquinic acid, caffeic acid 4-O-glucoside | P. abies, L. deciduas, P. sylvestris, T. baccata, P. mariana, P. pinaster, P. kesiya, L. deciduas, J. communis, P. menziesii, M. glyptostroboides | [18,21,39,41,42,49] |
| Stilbenes | trans-resveratrol, resveratrol, trans-pinosylvin, cis-stilbenes, pinosylvin, dihydro-monomethyl, trans-stilbenes, trans-piceatannol, trans-piceid, trans-isorhapontin, trans-isorhapontigenin, phenanthrenes, astringin, trans-astringin | P. mariana, P. abies, J. communis, P. pinaster, P. sylvestris, P. strobes, P. roxburghii, P. wallichiana, P. gerardiana, P. merkusii | [8,18,39,51,52,53,54,55,56,57] |
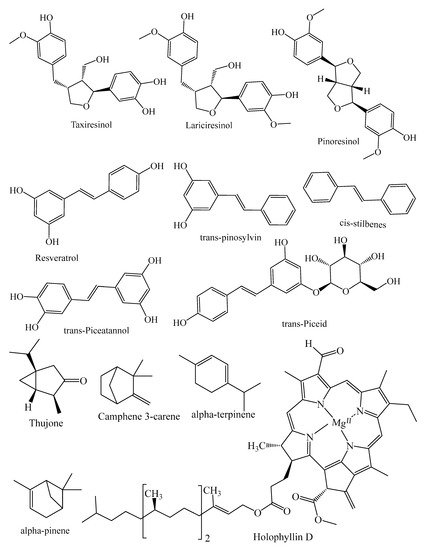
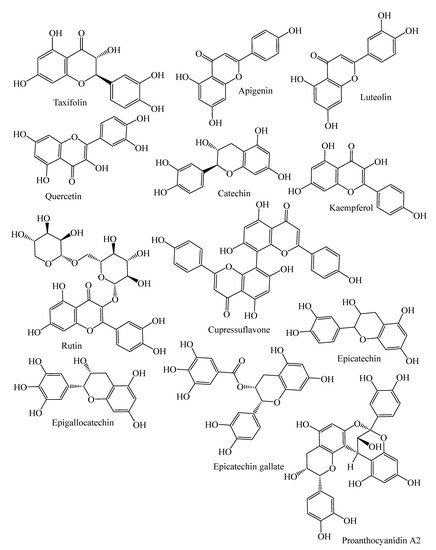
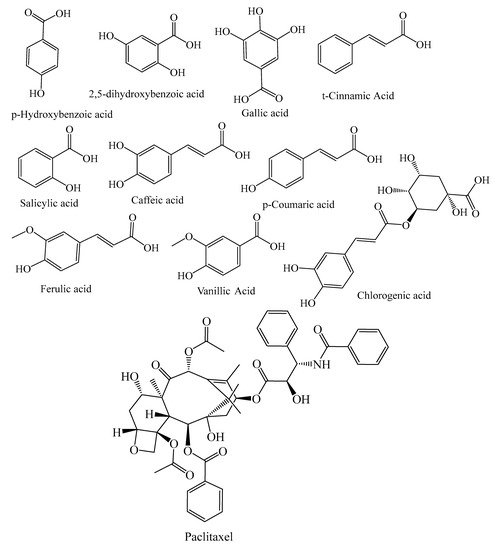
2. Conifers Phytochemicals Components
2.1. Terpenes
2.2. Alkaloids
2.3. Polyphenols
2.3.1. Flavonoids
2.3.2. Lignans
2.3.3. Stilbenes
2.3.4. Tannins
3. Traditional Medicinal Uses
4. Conifers Extracts Rich in Phytochemical with Putative Health Effects
4.1. Oxidative Stress
4.2. Synergism between ROS and other Diseases
4.2.1. Antioxidant Activity
4.2.2. Anti-Inflammatory Activity
4.2.3. Anticancer Activity
| Conifer spp. | Part Used | Compounds | Nature of Extract | Radical Scavenging Assay | Dose/Concentration | Main Effects | References |
|---|---|---|---|---|---|---|---|
| Aurocaria cookii | Leaves | Phenolic compounds | Methanol, chloroform, petroleum ether | DPPH | 1000 μg/mL | Methanol extract shows the best antioxidant activity with 63% inhibition, higher than the other two compounds | [147] |
| A. excelsa | Needle | Flavanoids | Methanol | DPPH | 50–72.5 μg/mL | Methanol/water extract shows antioxidant activity | [38] |
| C. deodara | Heart wood | Tannins, flavonoids, and phenolic compounds | Water/alcohol | DPPH, superoxide radical-scavenging activity, ABTS | DPPH-IC50 (μg/mL): 61.89 (water extract), 75.79 (alcohol extract) superoxide radical-scavenging activity— IC50 (μg/mL): 87.76 (water extract), 121.55 (alcohol extract). ABTS-IC50 (μg/mL): 115.29 (water extract), 122.42 (alcohol extract). |
DPPH radical-scavenging activity and the reducing power of C. deodara were potent in water and alcohol extract | [148] |
| C. japonica | All parts | Phenolic compounds | Methanol | ORAC, SOD |
4.09–7.64 TE/mg 3.63–4.06μg/mL |
The methanol extracts from each part of C. japonica except for pollen showed strong activities in the bioactivity assays. | [149] |
| J. communis | Berry | Flavanoids (quercetin rutin, apigenin) chlorogenic acid |
Alcohol/Water | DPPH | EC50 1.42 mg/mL against standard Ascorbic acidEC50 value of 0.365 mg/mL | The antioxidant activity was confirmed as 81.63 ± 0.38% by the DPPH assay. | [42] |
| L. laricina | Bark | Phenolic compounds | Ethanol/Water | ORAC | IC50 0.878 μg/mL. | Bark extract of LL shows significant antioxidant activity | [51] |
| Metasequoia glyptostroboides | Cone | Terpenoid | Ethyl acetate | DPPH, NO, superoxide, and H2O2 | 5–250 μg /mL | Sugiol derived from cone extract show good antioxidant activity—78.38, 72.42, 74.45 and 85.04%, respectively. | [26] |
| Picea abies | Bark | Atilbenoids | Ethanol/Water | DPPH | 49.74 μg/mL | UVA-induced modification of the stilbene-rich inner bark extracts increased the antioxidant activity as UVA irradiation decreased the capacity of the extracts to prevent lipid oxidation in the liposome system method | [53] |
| P. smithiana | Leaves | Phenolic compounds | Methanol | DPPH | IC50 (μg/mL)- | Results of the DPPH radical scavenging activity and FRAP study determine that methanol extracts of leaf displayed the highest antiradical efficiency | [150] |
| 228 | |||||||
| FRAP | 494 | ||||||
| Reducing Power assay | 978 | ||||||
| Pinus gerardiana | Bark | Phenolic compounds | Ethanol | DPPH | IC50 value μg/mL | P. gerardiana shows promising H2O2 radical scavenging activity | [104] |
| 102.8 | |||||||
| H2O2 | 81.83 | ||||||
| NO2 | 109.2 | ||||||
| P. halepensis | Bark | Phenolic compounds | Ethanol/Water | IC50 (μg/mL). Ethanol and the water | Ethanol and water extract of bark exhibited significant free radical neutralization capacities, at conc. 0.5–8 μg/mL | [151] | |
| DPPH | 3.28, 3.26 | ||||||
| ABTS | 3.1, 3.59 | ||||||
| P. pinaster | Bark | Phenolic compounds | Ethanol/Water | PB (50%) and (90%) IC50 value μg/mL | PP bark extracts formed from PB 50% (50% ethanol) have maximum (DPPH, ABTS) radical scavenging activity while FRAP shows activity with (PB 90%) | [39] | |
| DPPH | 49.74 | ||||||
| ABTS | 59.41 | ||||||
| FRAP | 101.3 | ||||||
| P. roxburghii | Bark | Phenolic compounds | Ethanol | IC50 value μg/mL | Pine extract shows significant antioxidant activity | [104] | |
| DPPH | 97.54 | ||||||
| H2O2 | 86.90 | ||||||
| NO2 | 111.38 | ||||||
| P. wallichiana | Bark | Phenolic compounds | Ethanol | IC50 (μg/mL) | Pine extract shows significant radical scavenging activity | [104] | |
| DPPH | 111.40 | ||||||
| H2O2 | 84.18 | ||||||
| NO2 | 98.5 | ||||||
| Thuja occidentalis | Leaves | Flavonoids, phenols | Methanol | DPPH, FRAP | 20–100 μg/mL | Crude extract shows significant antioxidant activity | [152] |
| T. occidentalis | Non-woody branches with leaves |
Polyphenol, flavonoids | Mother tincture (MT) | DPPH, ORAC, NO | 25 or 50 mg/kg | T. occidentalis mother tincture displayed 88.3% antioxidant activity by DPPH and about 78% by NO assay | [126] |
| Taxus baccata | Leaves and cones |
Flavonoids, phenols | Methanol | DPPH | IC50 (μg/mL) 105.41, 518.51 leaves and cones resp. | Acetone and ethyl acetate extract of leaves show good scavenging activity | [153] |
| Water | DPPH | 533.66, >1000 leaves and cones resp. | |||||
| Acetone | DPPH | 25.24, 81.43 leaves and cones resp. | |||||
| Ethyl acetate | DPPH | 29.84, 180.26 leaves and cones resp. | |||||
| Petroleum ether | DPPH | 438.92, > 1000 leaves and cones resp. | |||||
| T. wallichiana | Leaves | Terpenoids, flavonoids | IC50 values (μg/mL) | The maximum DPPH activity was observed in methanol extract (91.25%), followed by water (87.64%), ethanol (85.23%), and ethyl acetate (83.27%) at the highest concentration (700μg/ml) | [154] | ||
| Methanol | Superoxide radical | 170.30 | |||||
| DPPH | 212.00 | ||||||
| LPO | 126.09 | ||||||
| Hydroxyl radical | 82.34 | ||||||
| Ethyl acetate | Superoxide radical | 297.55 | |||||
| DPPH | 301.80 | ||||||
| LPO | 151.96 | ||||||
| Hydroxyl radical | 199.05 | ||||||
| Water | Superoxide radical | 257.00 | |||||
| DPPH | 258.29 | ||||||
| Hydroxyl radical | 175.33 | ||||||
| T. wallichiana | Leaf, stem | Polyphenols, flavanoids, terpenoids | Methanol | DPPH FRAP |
IC50 value (μg/mL.) Leaves (23.18) Stem (56.75) |
DPPH and FRAP activity of TW leaves and stem extract have high antioxidant activities. | [155] |
| Conifer spp. | Part Used | Nature of Extract | Compounds | Major Method(s) of Testing | Dose. Conc | Main Effect | References |
|---|---|---|---|---|---|---|---|
| Abies chensiensis | Twigs and leaves | Ethanol | Terpenoids | Induce lipopolysaccharide to produce inflammation in RAW 264.7 macrophage cells | 0.2–50.0 μM | 4 compounds—3α-hydroxyl-8,14,22Z,24-tetraenlanosta-26,23-olide; (5R,20R)-8(14→13R)-abeo-17,13-friedo-3-oxolanosta-8,14(30),22Z,24-tetraen-26,23-olide; 8,14,22Z,24-tetraen-3-oxolanosta-26,23-olide; and (23R, 25R)-3,4-seco-9β H-lanosta-4 (28),7-dien-16α-hydroxyl-26,23-olid-3-oate—extracted from extracts showed significant anti-inflammatory activities of inhibition against NO formation with IC50 value of 15.9, 18.7, 20.18, and 10.9 μM |
[125] |
| A. georgei | Aerial parts | Chloroform, ethyl acetate, n-butanol | Flavanoids | dimethylbenzene-induced ear oedema in mice | 200 mg/kg | AG ethyl acetate extract shows 18% inhibition against dimethylbenzene-induced ear edema in mice while carrageenin-induced paw edema in rats shows inhibition ratios 28.2% and 35.6%, after 2 and 6h, respectively. | [156] |
| Carrageenin-induced paw oedema rat | 140 mg/kg | ||||||
| A. webbiana | Leaves | Methanol/Petroleum ether extract | Flavanoids | Carrageenan-induced rat hind paw edema model in Albino mice | 400 mg/kg | Plant leaves extract possesses significant anti-inflammatory properties | [157] |
| Agathis robusta | Leaves | Methanol | Flavanoids, tannins and saponins | Heat induced hemolytic method in human red blood cell (HRBC) membrane | 400 μg/kg | Leaves extract shows good antiinflammatory activity | [158] |
| Cedrus deodara | Stem bark | Methanol | Deodarin, quercetin, taxifolin | Carrageenin-induced paw edema in Albino rat | 100 mg/kg | Anti-inflammatory activity with 43.47% inhibition | [159] |
| Cupressus macrocarpa | Leaves | Methanol | Cupressuflavone (CUF) | Carrageenan-induced paw edema model in Mice | 40, 80, and 160 mL/kg |
CUF demonstrated antiinflammatory activity by inhibiting paw edema with 55, 60, and 64%, by decreasing the plasma pro-inflammatory mediators PGE2, IL-6, TNF-a and IL-1b |
[46] |
| Juniperus communis | Berry | Alcohol/Water | Flavanoids (quercetin rutin, apigenin) chlorogenic acid | Acute-dextran and kaolin subacute inflammation induced in Wistar Rat | 10 mL/kg | The antiinflammatory action of the juniper extract, administered as a microemulsion in acute-dextran model was increased when compared to kaolin subacute inflammation induced model. | [42] |
| J. oxycedrus | Berry | Ethanol, n-butanol | Flavonoids (amentoflavone, cupressuflavone, hinokiflavone, and rutin) | Carrageenan-induced hind paw edema model in mice | 100 mg/kg | Ethanol extract of Joso berries displayed remarkable inflammatory inhibition ranging between 24.5% and 23.7% at 100 mg/kg in carrageenan-induced edema model | [160] |
| J. foetidissima | Berry | Ethanol | Flavonoids (amentoflavone, cupressuflavone, hinokiflavone, and rutin) | carrageenan-induced hind paw edema model in mice | 100 mg/kg | JFB extract at a dose of 100 mg/kg. shows high antiinflammatory effect 26.9% | [160] |
| Pinus gerardiana, P. roxburghii, P. wallichiana | Bark | Ethanol | Flavanoid, tannin | against albumin denaturation, HRBC membrane stabilization assay | 2500 μg/mL | P. roxburghii extract showed highest (%) of inhibition and protection i.e 86.54 and 89.92 against albumin denaturation and HRBC membrane stabilization. However, P. wallichiana have least inhibition and protection percentage, i.e., 76.54 and 81.2% | [104] |
| Taxus baccata | Aerial parts | Methanol | Terpenoids | ear edema induced in mice | 3.2 mg/ear | T. baccata extract displayed best activity | [21] |
| T. baccata | Bark | Ethanol | Alkaloids, terpenoids, flavonoids | carrageenan-induced paw edema in Wistar Albino rat | 200 mg/kg | Percentage of inhibition is 44% at a dose of 200 mg/kg | [161] |
| T. baccata | Heart wood | Ethanol | Taxoids, lignans | carrageenan-induced hind paw edema model inS wiss albino mice | 30–100 mg/kg | TBW shows significant antinociceptive and anti-inflammatory activities | [29] |
| T. wallichiana | Bark | Methanol | Tasumatrol B, 1,13-diacetyl-10-deacetylbaccatin III (10-DAD) and 4-deacetylbaccatin III (4-DAB) | carrageenan-induced paw edema and Cotton-pellet oedema model in Wistar rats and Swiss albino mice | 20 and 40 mg/kg; 40 mg/kg | In a carrageenan-induced inflammation model, tasumatrol B at a dose of 20 mg/kg showed significant activity, while in a cotton-pellet edema model tasumatrol B was found to be highly significant at the dose of 40 mg/kg. | [22] |
| Thuja occidentalis | Non-woody branches with leaves | Mother tincture (MT) | Polyphenols, flavonoids | Administered 2,4,6-trinitrobenzenesulfonic acid to induce intrarectal colitis in mice | 25 or 50 mg/kg | MT manage to relieve intestinal inflammation experimentally induce by TNBS in 7 days. | [126] |
| Conifer spp. | Part Used | Nature of Extract | Compounds | In Vitro and in Vivo Model | Dose. Conc | Main Effects | References |
|---|---|---|---|---|---|---|---|
| Abies georgei | Aerial parts | Chloroform, ethyl acetate, n-butanol | Flavanoids | Human tumor cell lines-A549, QGY-7703, LOVO, 6T-CEM | 77.5, 11.1, 7.8, 32.8 μg/mL | AGC extract has potent tumour and antiproliferative effects in humor tumor cell lines | [156] |
| (Mice) S180 tumours cell lines | 100, 200 and 400 mg/kg | AGC also exhibited activity in tumour growth inhibition in a dose-dependent manner, with ratios of 46.7, 53.1 and 31.0% at doses of 100, 200 and 400 mg/kg, respectively | |||||
| Araucaria angustifolia | Female strobili | Water | Fatty acids and polyphenols | Laryngeal carcinoma HEp-2 cells | 100–500 μg/mL | AAE inhibit the activity of mitochondria complex I and induce redox stress and cytochrome c, which leads cleavage of nuclear proteins of larynx HEp-2 cancer cells | [162] |
| Cedrus deodara | Stem wood | Chloroform | Lignans (Matairesinol, dibenzylbutyrolactol, (−)-Wikstromol) | In vitro human cell lines (cervix, breast, colon, liver, CNS, prostrate) | In vitro cytotoxicity IC 50 value-Wikstromol (71.31–93.63) and Matairesinol (50.84–95.36) μg/mL | CD lignin mixture have potent to show a cytotoxic effect at the maximum in CNS and at the minimum in liver against cancer cell lines in a dose-dependent manner at 100 μg/mL from 49 to 95%. | [34] |
| Human T lymphoblast, acute lymphoblastic leukemia cell line, Molt-4 and human promyelocytic leukemia cell line (HL-60) | IC50 (μg/mL) 15 |
AP9-cd-induced endogenous NO production leads to the generation of peroxide and disruption of mitochondrial membrane potential, leading to apoptotic pathway activation Increase in sub-G0 fraction from 35 to 60% in 24 to 48h |
[163] | ||||
| In vivo swiss albino mice (K562 cells) | The lignin mixture displays anti-cancer effects by regulating annexin V binding, intracellular caspase activities and DNA fragmentation | ||||||
| C. deodara | Needle | Ethanol | Kaempferol, myricetin, isorhamnetin and quercetin | HepG2 cells | IC50 114.12 μg/mL | TFPNCD shows potent cytotoxicity by inhibiting the growth of HepG2 cells in a dose-dependent manner Regulates cell cycle and apoptosis |
[164] |
| Cryptomeria japonica | Leaves | Methanol | Flavonoids | Albino mice of Ehrlich Ascites Carcinoma (EAC). | 100–400 μg/gm | Tumor cell count as well as the amounts of ascetic tumour cells in packed cells were significantly reduced in infected mice treated with MC | [165] |
| Juniper communis | Berry | Methanol and water | Phenolic compounds | CaCo2 and HeLa carcinoma cell lines | IC50 1300–2500 μg/mL | Methanol and water extracts of JCB show potent antiproliferative activity against cancer cell lines | [166] |
| J. taxifolia | Leaves | Chloroform | Polyphenols and lignan | human leukemia (HL-60) cells | 2.5 μg/mL | 7α-hydroxysandaracopimaric acid, a diterpenoid compound obtained from J. taxifolia leaves, shows antitumor effects on HL-60 cells | [24] |
| J. phoenicea | Aerial parts | Chloroform | Polyphenols | IC50 values (μg/mL) | It is found that JPCF disrupts cell cycle progression in the G0/G1phase and shows apoptotic, antiproliferative and necrotic effects on cancer cells lines | [20] | |
| Human lung (A549) | 34.2 | ||||||
| Breast (MCF-7) | 24.5 | ||||||
| Liver (HepG2) cancer cells | 57.6 | ||||||
| J. foetidissima | Needle | Methanol | Quercetin, rutin | Rat brain tumor (C6) cell lines | IC50 values (μg/mL) 10.65 |
J. foetidissima needle extract showed significant antiproliferative activity | [50] |
| M. glyptostroboides | Leaf | Water | Polyphenols | PC12 cells | 25 μg/mL | M. glyptostroboides leaf extract shows a cytotoxic effect and prevents oxidative damage of neuronal PC12 cells, protecting them from apoptosis; it was also found to significantly inhibit the release of LDH, which may result from apoptosis or necrosis | [49] |
| Picea wilsonii | Whole plant | DMSO | ND | Human keratinocyte HaCaT cell lines | 1–3 g/mL | PwM extracts inhibit the production of MCP-1 IL-6, IL-13 and but do not inhibit IL-8 production | [115] |
| Pinus kesiya | Woody twig | Ethanol | Phenolic compounds and flavonoids | Human hepatocarcinoma (HepG2) cell lines | IC50 (μg/mL) 52.0 | PK Extract exhibited a potent cytotoxic effect in the HepG2 cell line | [167] |
| P. kesiya | Branch | Ethanol | Phenolic compounds and flavonoids | Human leukemic U937 cancer cells |
IC50: 299 μg/mL | PK ethanol extract possesses anticancer activity against U937 human leukemic cells via apoptosis | [168] |
| P. merkusii | Leaves | Methanol | Phenolic compounds | MCF-7, A549, HT 1080 and HepG2 Huh-7 cancer cell lines | IC50 (μg/mL) 4.5, 16, 4.1, 5.6, 9.5 |
PM methanol extract possesses anticancer activity against human cancer cell lines | [169] |
| T. baccata | Leaves, cones | Methanol | Phenolic compounds | HCT-116 human colon cancer and MDA-MB-231 human breast cancer cell lines | IC50 μg/mL Leaves: 14.43 and 4.59 cones: 49.69 and 133.53 |
Methanol extracts of leaves had better activity on HCT-116 cells than seed cone extract, with IC50 values of 14.3 for 24 h and 4.59 for 72 h. Meanwhile, extracts did not show any significant cytotoxic effects on the cancer cell lines | [153] |
| T. wallichiana | Heartwood | Methanol | Lignans 1 (taxiresinol 1) 2, 3 | colon, ovarian liver, and breast cancer cell lines | IC90 lignan 2 and 3 μg/mL Caco 2:0.08 and 0.056 and 0.251 |
Taxiresinol 1 shows anticancer activity against ovary, colon, liver and breast cancer cell lines, while lignans 2 and 3 were found to be most active against Caco-2 cell lines | [170] |
| T. yunnanensis | All parts | ND | α-Conidendrin | MCF-7 andMDA-MB-231 cancer cell lines | 40 μM | α-conidendrin have the potential to inhibit human breast cancer cell lines MDA-MB-231 and MCF-7, showing viability of 73 and 82%, respectively | [31] |
| P. roxburghii | Leaves | Water and ethanol | Phenolic compounds | A549 human lung cancer cell line | 111.2 and 112.7 μg/mL | PRL extract shows potent anticancer activity against cancer cell lines. | [171] |
| Taxus cuspidata | Branches and leaves | Water | Polysaccharides | MCF7 | IC50 μg/mL | Purified polysaccharides (Pe4) on HeLa cells had the highest inhibitory effect, and its IC50 value is 89.9, while (Pe1) shows the best cytotoxic capacity against cancer lines HepG2 and MCF7, with IC50 conc. 132.0 and 169.0 μg/mL, respectively | [172] |
| 169.0 | |||||||
| Hela | 89.9 | ||||||
| HepG2 | 132.0 | ||||||
| Thuja occidentalis | Leaves and non-woody branches | Mother tincture (MT) | Polyphenols including flavonoids | Caco-2 cells | 25 or 50 mg/kg | Caco-2 cells exposed to H2O2 and T. occidentalis MT proves its radical scavenging activity by reducing GSH level by 103% and 98% as compared to TNBS group; MT also managed to reduce the lipid peroxidation | [126] |
| T. occidentalis | Leaves | Ethanol | ND | Human NSCLC (A549) cell lines | IC50 μg/mL | Extract of TO shows both anticancer and antiproliferative activities against NSCLC (A549) cell lines in a dose-dependent manner. | [173] |
| 282 | |||||||
| Human normal embryonic cell lines (L-132) | 376 | ||||||
| T. occidentalis | ND | Mother tincture (MT) Thujone-rich fraction (TRF) |
Thujone | A375 human malignant melanoma cell line | 200 μg/mL | TRF as compared with TO MT on exposure to A375 cells exhibited highly cytotoxic, apoptotic and antiproliferative effects, but TRF shows a lower growth inhibitory response towards peripheral blood mononuclear cell (normal cells) | [174] |
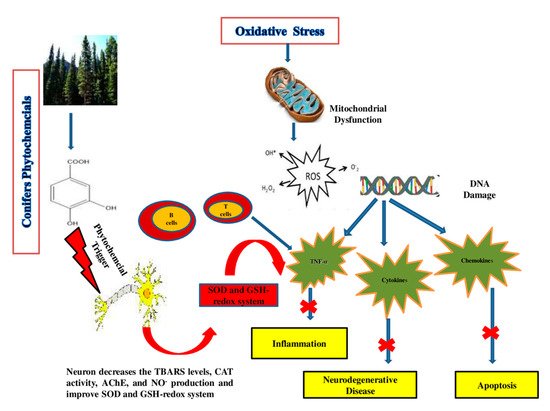
4.2.4. Neurodegenerative Diseases
| Conifers spp. | Compounds with Neuroprotective Potential | Model | Effective Concentration | Relevant Bioactivities | Reference |
|---|---|---|---|---|---|
| Abies holophylla | Holophyllin-D | C6 glioma cells | 20 μM | Diterpenes compound holophyllin D shows neuroprotective potential in C6 glioma cells by inducing nerve growth factor | [25] |
| Araucara angustifolia | Catechin, epicatechin and rutin | Rat | 10 mg/mL | AAE has antioxidant and neuroprotective properties as it decreases the TBARS levels, CAT activity and NO production in the hippocampus region of the brain in rats. | [235] |
| A. angustifolia | Catechin, epicatechin, rutin, quercetin and apigenin | human dopaminergic SH-SY5Y cells | 5 μg/mL | Decrease in the production of neuron (ROS) and lipid peroxidation. | [43] |
| A. angustifolia | Quercetin | cockroach | 200–400 μg/g | Neurotoxicity modulates the behavior of insects by altering the dopaminergic pathways, as quercetin has the ability to induce selective inhibitory actions on NMDA and GABA receptors and inhibit the enzyme acetylcholinesterase (AChE) | [236] |
| Cedrus deodara | Cedrin | PC12 cells | 0.1, 1 and 10 μM | PC12 cells injured by amyloid β1–42 can be improved by cedrin. Cedrin can reduce (ROS) overproduction, enhance the activity of SOD and decrease MDA content and inhibition of oxidative stress, improvement of mitochondrial dysfunction and suppression of apoptosis in PC12 cells | [237] |
| Metasequoia glyptostroboides | Gallic acid, rutin, myricetin, kaempferol, quercitrin, epigallocatechin, epicatechin gallate epigallocatechin gallate and caffeic acid | Neuronal PC12 cells | 2 mg/mL | The extracts effectively reduced the hydrogen peroxide-induced lipid peroxidation in neuronal PC12 cells by decreasing intracellular ROS accumulation | [49] |
| Pinus densiflora | Catechin, quercetin dehydrate, astragalin and kaempferol | Mice | 50–100 mg/kg | Catechin displayed a potential effect protecting mouse brains from oxidative damage via the improvement of the antioxidant capacities of TAC, the GSH-redox system, SOD and CAT in the hippocampus region as well as the inactivation of cytokines such as NF-kB in pyramidal cells of the hippocampal CA1 region, while PNE shows antiamnesic properties and effects in Alzheimer’s, as it attenuated the increase in serum corticosterone level and up-regulation of GR hippocampal gene expression | [238,239] |
| P. eldarica | Needle extract | Mice | 50 mg/kg | Alkanes, sterols, terpenoids, and quercetin, which is found in P. eldarica, help in inducing sleep and alter the sleep–wake cycle partly via activation of GABA receptors | [240] |
| P. massoniana | Polyprenols | Mice | 25 mg/kg | Polyprenols significantly increased T-AOC, GSHPx, damaging peroxide components from cells in order to stop the lipid peroxidation chain reaction and avoid excessive hydrolysis to form NEP, MDA, SOD activity (remove free radicals) and β-site AβPP cleaving enzyme 1 (BACE1) expression, while NOS activity, MDA concentration, NO, concentration of Aβ1-42 and PS1 were reduced | [241] |
| P. pinaster | Pycnogenol (PYC) | Mice | 20 mg/kg | In the MPTP-induced mouse model, PYC could prevent dopaminergic neurons by reducing oxidative loads, suppressing glial cell activation, and inhibiting inflammatory responses | [100,242] |
| P. roxburghii | Quercetin, rutin, gallic acid | Wistar albino | 100–300 mg/kg | Quercetin and gallic acid, both present in stem bark, have been shown to inhibit neuronal toxicity and apoptosis by reversing mitochondrial dysfunction and free radical development | [243] |
| Thuja occidentalis | Water extract | Mice | 100 mg/kg | CNS depressant activity, anticonvulsant and muscle relaxant activity | [244] |
| Torreya nucifera, | Arctigenin | Rat Cortical cells | 0.01 µM to 10.0 µM. | Arctigenin significantly attenuated glutamate-induced neurotoxicity by inhibiting the binding of [3H]-kainate to its receptors | [35] |
| T. semen | Polyphenols, flavonoids | Mice | 0–10 mg/mL | TS increased the level of total glutathiones | [245] |
4.2.5. Alzheimer’s Disease (AD)
4.2.6. Parkinson’s Disease
4.2.7. Insomnia
This entry is adapted from the peer-reviewed paper 10.3390/molecules26103005
