The non-ionizing and non-invasive nature of THz radiation, combined with its high sensitivity to water, has made THz imaging and spectroscopy highly attractive for in vivo biomedical applications for many years. Among them, the skin is primarily investigated due to the short penetration depth of THz waves caused by the high attenuation by water in biological samples. A complete model of skin describing the THz-skin interaction to reveal the optical properties of the skin from the measured THz spectrum is needed. It is crucial that the correct model is used, not just to ensure compatibility between different works, but more importantly to ensure the reliability of the data and conclusions. Therefore, in this review, we summarize the models applied to skin used in the THz regime, and we compare their adaptability, accuracy and limitations.
- Terahertz spectroscopy
- in vivo
- skin
- skin modeling
1. Introduction
1.1. Terahertz Radiation and Systems
Terahertz (THz) waves lie between 0.1 and 10 THz (1THz = 1012 Hz), corresponding to wavelengths ranging from 30 μm to 3 mm. The rapid development of THz technology in the last three decades has promoted numerous applications in communication, security, biosensing, aerospace etc. Among them, biomedicine has long been considered a promising application area [1]. One important reason is the non-ionizing and non-invasive nature of THz radiation, making it a safe modality for biomedical in vivo imaging. Another key factor comes from the high absorption of water, which despite limiting the depth of penetration, provides high sensitivity to the water content in living tissues. Given these characteristics, THz in vivo studies have been mainly focused on skin, as THz waves can penetrate through the superficial layer, and the measured THz response is sensitive to its water concentration and tissue structure.
The aim of skin measurements is to investigate the morphology, histology, functions and properties [2]. Moreover, it is of great interest to apply skin imaging and measurements for the diagnosis of skin lesions and pathological processes objectively and quantitively [2,3]. A wide range of imaging methods are currently used. Histological examination of biopsies usually combined with optical microscopy is invasive and requires sample preparation and fixation which can change the biological properties of the sample but is the gold standard to reveal pathological changes in tissues [4]. Electron microscopy provides especially high resolution at nanometer level. However, sample preparation is required to enhance the contrast [5]. To meet the need for non-invasive in vivo measurements of skin, emerging techniques are now available or under research. Fluorescence microscopy is another kind of optical microscope based on fluorescence and phosphorescence and combined with confocal laser scanning microscopy, it has been widely used for evaluating transdermal drug delivery [6]. It is able to track and quantitatively analyze drugs labelled with fluorescent dyes. Near infrared(NIR) imaging is another commonly used method for skin measurement, it can also be used for hydration sensing because of the clear absorption of water molecules at 1450 and 1920 nm [7]. However, NIR imaging usually yields complex spectra which are difficult to interpret. Raman spectroscopy combined with confocal microscopy can provide information about the chemical components and concentration distribution through the depth of the skin, but it is limited by the slow imaging speed, low sensitivity, and sophisticated data analysis required [8,9]. Optical coherence tomography (OCT) is another method that can be used to measure the skin, but it primarily reveals morphological changes in the skin [10,11]. Computed tomography (CT) and magnetic resonance imaging (MRI) are other commercialized medical imaging techniques, however, they both have limitations. CT involves X-ray radiation which is ionizing [12,13] and MRI is best suited to imaging internal soft tissue though there is research investigating the application of this technique to skin imaging, but it is still at a preliminary stage [14]. Based on dielectric differences of normal and malignant tissues, microwave and millimeter-wave technologies also provide cost-effective options for tumor diagnosis [15,16]. It has been reported that millimeter waves are also sensitive to the water and thickness variation of skin and could be a potential technique for skin diagnosis [17,18]. Compared to THz waves, millimeter waves have a deeper penetration depth in living tissues of over 1 mm, reaching down to the dermis layer [17,18]. However, the longer wavelengths also restrict the spatial resolution limit for standard imaging configurations. Based on the sensitivity of THz radiation to water and its penetration depth (100 μm to several mm) into skin and tissues, THz sensing could provide superficial information and is therefore suitable for skin measurements. THz sensing probes the intermolecular vibrations of water and other biomolecules, while NIR measurements are dominated by intramolecular vibrations. Given the high sensitivity of THz light to water content, normal tissues and cancerous tissues can be differentiated. Moreover, the picosecond-level time-resolved ability of THz pulsed imaging enables a depth resolution of ~100 μm, comparable to 50–100 μm level of MRI [19]. Combined with appropriate skin modelling which will be detailed in Section 2, better depth resolutions down to few tens of micrometers could be achieved. Compared to OCT which mainly reveals the structure and morphology of tissues, THz imaging is sensitive to both the structural and chemical properties. Therefore, THz imaging is a promising technique for quantitative in vivo skin analysis, which could aid the diagnosis of skin lesions and pathological processes. However, various technical challenges need be overcome before THz techniques can be robustly adopted in a clinical setting. The current THz systems still suffer from low imaging speeds, limited measurement flexibility and critical optical alignment. The further development of THz devices and systems can gradually pave the way to its utility and acceptance in wider applications.
For THz in vivo skin measurements of humans, reflection geometry is required as tissues highly attenuate the THz radiation [20]. Various THz devices can be adopted to perform reflection measurements. For example, laser feedback interference in quantum cascade lasers is a promising technique for biomedical imaging [21]. Such a mechanism not only provides a high resolution due to the short wavelength (frequency typically > 2 THz), but also a good signal-to-noise ratio originating from the coherent nature of the interference. Rakić et al. have successfully employed this technique to image porcine tissues and murine skin [22,23]. Similar setups can be adapted to in vivo measurements. THz time-domain spectroscopy (TDS) is the most widely used technique for in vivo studies. Figure 1a shows a typical THz reflection-mode TDS system based on fiber-coupled photoconductive antennas. In this system, the femtosecond pulse from the fiber laser is split and sent to the THz emitter and detector, respectively. On the emitter side, the input femtosecond laser pulse excites the free carriers on the semiconductor substrate. The carriers are accelerated by the biased voltage on the electrode and quickly recombine in a few picoseconds. The rapidly generated and annihilated carriers form a transient current, which then radiates an electromagnetic wave with its electric field proportional to the time-variation of the current. This radiation thus contains broadband THz frequencies given by its picosecond pulse width. The THz wave is then guided by the optics, reflected by the sample and collected by the detector. In the detector, the femtosecond pulse again excites the photocarriers, which are accelerated by the THz electric field to produce the photocurrent. The generated photocurrent in detector is then amplified. As the femtosecond pulse is over an order shorter than the THz pulse, the detected current is only proportional to the THz electric field at the moment it interacts with the THz wave. By moving the delay stage to change the optical path difference between the pumping and probing light, the whole THz waveform can be sampled in the time-domain. Figure 1b illustrated the examples of the THz time-domain waveforms reflected from the quartz-volar forearm and quartz-air interfaces, respectively. In such a setup, the THz image can be acquired by raster scanning the region of interest by either moving the window-sample system or the optical system. Figure 1a shows the latter approach. For imaging data, any model that applies for the skin characterization is then applied to the data at each pixel.
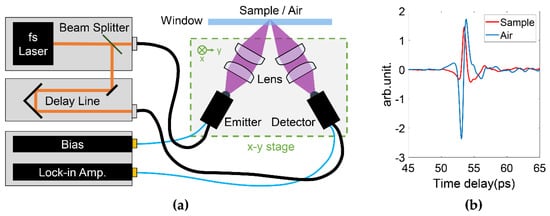
Figure 1. (a) Typical THz pulsed laser imaging system in reflection geometry. The THz optical system is assembled on a x-y 2D stage to enable raster scanning the sample. (b) Examples of the THz pulses reflected from the quartz-volar forearm and quartz-air interfaces, respectively.
1.2. Biomedical Applications of THz Imaging
As previously introduced, in vivo THz studies have mainly focused on skin due to the shallow penetration depth. Investigations into utilizing THz imaging for diagnosis of cancer, scar measurements, monitoring drug diffusion and hydration sensing have been reported [24,25,26,27,28]. The origin of these applications is mostly based on the sensitivity to water. For example, THz imaging was shown to be capable of identifying cancerous regions as the higher water content and the structural changes of tumors compared to healthy tissue leads to an increased refractive index and absorption coefficient [29,30,31]. Wallace et al. used THz imaging to identify basal cell carcinoma (BCC) and the results showed high correlation with histology images [32]. Other investigations have also demonstrated that THz imaging is able to detect the boundary of breast and brain tumors [30,31].
Utilizing the excellent depth-resolving ability of THz-TDS, early work by Cole et al. showed that a single THz pulse is able to identify the stratum corneum (SC), the upper layer of human skin, and measure the change in thickness across different regions on the body [33]. This is also enabled by the water-content difference between the SC and the lower epidermis, as the SC is normally much less hydrated. However, for skin in other body sites, such as the volar forearm and wrist, this is not the case as the SC is so thin that the second reflection cannot be resolved. Scars are also found to have different water concentrations from healthy tissue. Fan et al. used THz imaging to monitor the human scar healing process and observed that the difference in the optical properties of scarred and healthy tissue are still distinguishable even after few months. This means that THz imaging could help monitor scar treatment and management [34]. Further work by Wang et al. used THz spectroscopy and imaging to evaluate the effect of treating human skin with silicone gel sheeting. This work indicated that THz imaging is able to detect subtle fluidic changes inside skin [35].
Drug diffusion is also accompanied with changes of the water concentration inside skin. Kim et al. used THz reflection imaging to monitor the transdermal drug delivery of ketoprofen and DMSO mixtures and show that THz imaging is able to differentiate different concentrations of drug solution and that the pulse information can reveal the depth of drug penetration [36,37]. Wang et al. show that THz imaging could be used as a label-free modality to evaluate the efficiency of different transdermal drug delivery methods including needle patches. They also revealed that the changes in the THz signal are caused by the drug solution displacing water inside skin, this means it is possible to extract the amount of drug solution that has penetrated into the skin [26].
1.3. Variables Affecting In Vivo THz Measurements of Skin
In vivo measurements are a lot more complicated than ex vivo measurements, due to the complexity of living tissues, variations between different subjects, changes in the individual conditions etc. Variables that will affect the THz response should be carefully considered and well controlled during the measurement. Therefore, it is of vital importance to employ a robust experimental protocol to enable consistent in vivo measurements. For example, skin measurements are usually conducted in a reflection geometry with a window, either made of quartz or polymer, to help position the skin. Therefore, the contact pressure and occlusion by imaging window inevitably affect the result. Wang et al. found that the contact pressure between skin and the quartz window can significantly alter skin properties. A higher pressure applied to skin usually leads to a lower reflectance [38]. Another factor that needs to be considered is the occlusion effect. When skin is in contact with a window, water molecules can no longer evaporate to the outside of skin and accumulate in the SC, the water hydration inside the skin therefore increases. Sun et al. report how occlusion affects skin measurements and apply a biexponential model to describe the occlusion effect, making it possible to account for changes during raster scanning due to occlusion [39].
1.4. Aim of this review
The ability to use THz imaging and spectroscopy for different skin applications has been demonstrated through many studies. However, a unified model of skin for use in the THz range that unambiguously interprets the light-skin interactions has not yet been found, partially due to the complexity of living tissues and the divergent measurement protocols. Therefore, we present an overview of the applications of THz sensing of human skin with a focus on the model of skin used, and compare the adaptability, accuracy and limitations of these models. The models in this review consider the skin structure as a function of depth: the spatial variation in 2D imaging scan is not within the scope of this review.
2. THz measurement and modeling of skin
In this section, we focus on the optical models used in the THz regime for skin, including the dielectric model for describing the optical properties, and the structural models that describe the light-skin interaction. The former is related to the polarizing properties of different tissues of skin in response to the THz electromagnetic radiation. It is not necessary in every skin characterization, but in many cases it can be very useful to represent the optical properties by a model with fewer unknown parameters. In contrast, the latter which describes how the physical structure of skin is perceived by THz waves, is essential to convert the THz field information to skin-related parameters. THz waves can probe the structure of skin such as the SC and epidermis based on the clear differences between the two layers. As determined by Confocal Raman spectroscopy, the SC has a depth-dependent water concentration gradient and the epidermis has a more constant value[56]. Therefore models further separating the SC into multiple layers of different water fractions have been proposed. Based on different assumptions of water gradient changes inside skin, several structural models were proposed with dielectric models to relate the water gradient with optical indices. However, the layered cellular structure originating from the flattened corneocytes in the SC induces anisotropy which is polarization sensitive and can also be probed in the THz regime. Therefore, we totally overview four structural models reported in the literature. Whether to combine a skin model with a dielectric model or not is a trade-off problem: between the model accuracy and result accuracy, and there is a large divergence between different approaches. For example, establishing a comprehensive and precise model of skin may provide an accurate description about the light-skin interaction, but may result in too many unknown parameters that cannot be solved unambiguously. Combining various models to simplify this may reduce the credibility of the results, as errors in each model could be propagated and summed up. Therefore, a comprehensive overview and discussion of these models is necessary for the advancement of THz in vivo studies of skin. Details of the models can be found in this review.
3. Future Perspective
The variety of models and measurement protocols results in divergent results in different THz in vivo skin measurements, creating obstacles for comparisons between different studies. For example, as mentioned in Figure 4, by using different biological background refractive indices, the extracted water concentration can be different. Using a consistent measurement protocol is another important factor to ensure that results can be meaningfully compared [40]. Variables such as applied pressure and occlusion time should be carefully controlled as they significantly affect the reflectivity. Due to the occlusion effect rapidly changing the water concentration with time, current in vivo studies of skin are mostly point scans or line-scans [38,53]. By developing robust protocols, we can also overcome difficulties in comparing results taken on a variety of setups, such as with different angles of incident, polarizations, bandwidth. Faster, more accurate THz systems are needed before the skin models can be applied to interpret more complex applications such as drug diffusion along the vertical plane. Indeed, advances in single-pixel THz cameras are likely to pave the way for real applications [45]. Moreover, human skin is very diverse. Age, gender, and ethnicity could also be important factors that result in inconsistency. It has been reported that human skin of different ethnic types shows clear differences in structure and function [61]. For example, Asian skin in general shows higher water contents and higher SC lipid levels [61]. Studies have shown that aging skin shows decreased epidermis thickness [62], is more susceptible to become dry in low-humidity environments and is often characterized by roughness and wrinkling [63]. However, studies on the hydration levels in different genders do not show much relation. Gender differences have been investigated by Firooz et al., which showed slightly higher hydration in the female group but not statistically significant [64] while studies by Ehlers et al. [65] and Wilhelm et al. [66] showed no correlation between skin hydration and sex. These factors may also affect THz the THz response. Barker et al. demonstrated the clear difference in THz pulse for Asian male and Caucasian male skin [67]. Peralta et al. measured the THz optical differences during melanogenesis using in vitro skin models from Asian, Black, and Caucasian races [68]. However, there are still limited studies on the influence of different skin types. Our current research bypasses the need to quantify parameters for each skin type by measuring a “control region” on any subject as well as a “treated region”. This approach can be extended to investigating skin conditions too, and accounts for environmental factors which also affect the skin's response.
Apart from consistent THz models and measurement protocols, there is also demand for a robust algorithm for parameter extraction, especially as the number of unknown parameters grows. This is not an issue in the single layer structure when it is a simple two-parameter optimization problem. However, when the number of fitting parameters goes beyond 5, classical iteration optimizations may require a precise estimation of the initial values to ensure the convergence. In most cases, the computational complexity would be too large for these algorithms to handle. In this case, heuristic algorithms, optimization methods frequently used for multi-dimensional optimization problems, can be used to balance the accuracy and complexity. For example, the optimization of double Debye parameters has been achieved by using genetic algorithm (GA) by Clegg et al. [69,70] and Ding et al. [71], using a branch and bounding (BB) method by Bao et al. [72,73], and using particle swarm optimization by Yang et al. [74]. A GA was also adopted in the anisotropic SC model fitting by Chen et al. [53]. These algorithms can be efficiently utilized in extracting multiple parameters in a comprehensive skin model.
This entry is adapted from the peer-reviewed paper 10.3390/s21113624
