High density lipoproteins (HDLs) are commonly known for their anti‑atherogenic properties that include functions such as the promotion of cholesterol efflux and reverse cholesterol transport, as well as antioxidant and anti‑inflammatory activities. However, because of some chronic inflammatory diseases, such as type 2 diabetes mellitus (T2DM), significant changes occur in HDLs in terms of both structure and composition. These alterations lead to the loss of HDLs’ physiological functions, to transformation into dysfunctional lipoproteins, and to increased risk of cardiovascular disease (CVD).
Background
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by defective insulin secretion and reduced tissue sensitivity to insulin, leading to hyperglycemia. Mortality and morbidity resulting from DM are consequences of long-term micro and macrovascular complications. The increasing prevalence of DM worldwide represents an important public health problem. The latest edition of the International Diabetes Atlas showed that 463 million adults live with DM and that more than 4 million people aged between 20 and 79 years died from causes related to DM in 2019. In addition, estimates show that there will be 578 million individuals affected by 2030 and 700 million in 2045 [1]. Type 2 diabetes mellitus (T2DM), characterized by an increase in blood glucose as a result of progressive increase in insulin resistance and pancreatic incompetence to meet the progressive demand for insulin production, corresponds to about 90% of all cases of DM and its prevalence is increasing worldwide. T2DM and insulin resistance are also well known for significantly increasing cardiovascular (CV) risk. Notably, in individuals with T2DM, an increase of 2 to 4% in mortality from coronary artery disease is estimated [2]. The dysfunction of pancreatic beta cells, a critical component for the pathogenesis of T2DM, has been attributed to glucotoxicity and high levels of free fatty acids with a high inflammatory response. An additional possible pathogenic mechanism is that hyperglycemia accelerates atherogenesis by increasing the oxidation of lipoproteins.
Alterations of HDL Plasma Level and Composition in T2DM
It is well established that T2DM is associated with quali-quantitative changes in circulating lipoproteins. The lipoprotein profile of dyslipidemic, diabetic subjects includes an increase in triglycerides (TGs) and apolipoproteinB (ApoB)-containing lipoproteins (especially small and dense low-density lipoproteins (LDLs) and very low-density lipoproteins (VLDLs)) and a decrease in HDL levels. In addition, HDL structure and composition are significantly perturbed, with abnormal enrichment in TGs and depletion of cholesterol and ApoA-I [
13]. Thus, the phenotype of HDL in T2DM is the result of both reduced levels of circulating particles, and alterations, either as an increase or decrease, in crucial structural components. In the following paragraphs, we detail the compositional and functional characteristics of these abnormal HDLs ().
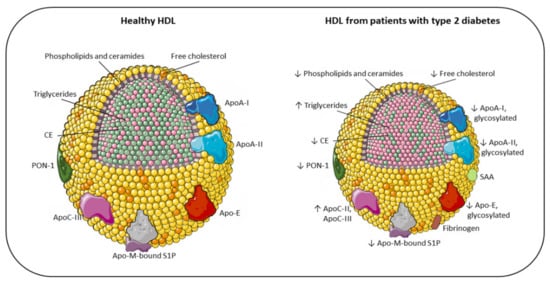
Figure 1. Main compositional differences between the HDL of healthy individuals and the HDL of patients with type 2 diabetes. Apo: apolipoprotein; CE: cholesteryl ester; PON-1: paraoxonase-1; S1P: sphingosine 1-phosphate; SAA: serum amyloid A; TG: triglyceride; ↓ decreased; ↑ increased.
Schematic representation of the main changes in HDL composition due to T2DM. HDL size of diabetic patients is altered, with loss of large and very large HDL
2 and with a shift toward small HDL
3. Accordingly, the lipid core is modified, with an enrichment in triglycerides (pink) instead of CE [
14], resulting in less stability and higher renal elimination [
15]. As a consequence of the reduction in surface lipids (phosphatidylcholine, ether-linked phosphatidylcholine, sphingomyelin, ceramides and free cholesterol), HDLs in diabetes are characterized by an alteration of their architecture and fluidity. Additionally, the content of proteins in HDLs is significantly altered in T2DM, with an increased SAA, fibrinogen, ApoC-II, and ApoC-III levels, and a reduction in ApoA-I, ApoA-II, ApoE, ApoM, and PON-1. Moreover, because of hyperglycemia and oxidative stress, diabetic patients present a large array of glycosylated proteins [
16]. All these compositional changes have a significant impact on the antiatherogenic functions of HDL, resulting in impaired antioxidant, anti-inflammatory, and vasodilator activity, together with a reduction in HDL cholesterol efflux capacity in patients with T2DM [
17,
18,
19,
20].
2.1. Modifications of HDL-C Plasma Levels
Dyslipidemia is a peculiar feature of individuals affected by T2DM and it is observed in 60–70% of patients [
21]. Despite the observation that the appearance of low levels of HDL-cholesterol (HDL-C) levels may precede the onset of DM, the direction of the causal relationship between diabetes and reduced HDL is still a matter of debate. The hypothesis of low HDL-C levels as a consequence of diabetes is supported by the evidence that, in a setting of insulin resistance, elevated plasma TGs may drive a cholesteryl ester transfer protein (CETP)-mediated mechanism leading to the formation of cholesteryl ester (CE)-depleted, small HDL that are rapidly catabolized by the kidneys [
15]. On the other hand, pre-existing low HDL-C levels may facilitate the onset of diabetes and its complications through the loss of protective functions on pancreatic beta cells [
22] or on endothelial cells [
23]. Epidemiological studies have consistently shown that low plasma levels of HDL-C are inversely related to the risk of T2DM development [
24,
25] and that they are independent predictors of diabetes complications such as the amputation of lower extremities, wound-related mortality [
26], and diabetic nephropathy [
27].
This controversy is not resolved by genetic studies, which provide conflicting results on the relationship between HDL and T2DM. While some reports suggest that a genetic predisposition to low HDL predicts an elevated risk of T2DM [
28], others, based on a Mendelian randomization approach, did not support such an association [
24].
2.2. Modifications of HDL Size
Seminal studies employing density ultracentrifugation techniques revealed significant perturbations of HDL size in diabetic individuals, with the loss of large and very large HDL
2 and the gain of small HDL
3, rich in TGs and poor in cholesterol [
29]. Consistently, two-dimensional gel separation studies have shown that diabetic subjects exhibit lower levels of large α-1, α-2, and pre-α-1 particles and higher levels of lipid-poor α-3 HDLs [
30]. Using the nuclear magnetic resonance (NMR) spectroscopy method, it has been demonstrated that subjects with diabetes present reduced levels of medium (9.0–11.5 nm) and large (11.5–18.9 nm) HDL particles but enriched small (7.8–9.0 nm) HDL compared to controls [
31]. Interestingly, it seems that particle size shifts in HDLs may precede the diagnosis of T2DM, as suggested in a study that found that levels of small HDL particles were positively associated with future T2DM risk, whereas large HDL particles showed an inverse association [
32]. This remodeling is mainly driven by the above-mentioned raise in CETP activity, leading to increased production of TG-enriched HDLs. These particles represent the optimal substrate for endothelial and hepatic lipases that promote the production of small, dense HDLs [
33]. In addition, the shift in HDL size can also be attributed to impaired LCAT activity due to high levels of glycated HDL (see below), which represent poor substrates for this enzyme [
34,
35].
In the context of HDL size changes, it is worth remarking that a “gold standard” separation method for HDL subclasses is still lacking [
36] and that further studies are needed to univocally conclude on which subclasses of these particles are most related to their atheroprotective functions, even in the T2DM setting.
2.3. Modifications of the Lipid Content
As previously cited, the increase in TGs and decrease in CEs in HDLs is a hallmark of T2DM, and it has been established for years. This lower CE/TG ratio, by conferring less stability than normal particles, is likely to be responsible for the higher susceptibility to renal elimination [
37]. In recent years, the application of mass spectrometry and NMR-based techniques has allowed the full characterization of the HDL lipidome in health and disease. In particular, two studies [
38,
39] showed consistent results on plasma lipid distribution in dyslipidemic, diabetic individuals and demonstrated that most species of lipids were present in significantly lower concentrations, whereas only a minority was increased compared to HDLs from control subjects [
38]. In detail, surface lipids such as phosphatidylcholine, ether-linked phosphatidylcholine, sphingomyelin, ceramides, and free cholesterol (FC) were reduced between 10 and 50% in individuals with T2DM and dyslipidemia [
39]. Since surface lipids are components that determine the architecture of HDL particles, such as the degree of surface fluidity, it is expected that these alterations may impair the properties related to their function as an acceptor of cholesterol and anti-inflammatory or anti-oxidant activity [
13,
39]. In addition, the lipid content of the HDL core showed a reduction in CEs (−8%) and an increase in the absolute amounts of TGs and diacylglycerol (DG) (+77%) in diabetic compared to control particles [
39].
Interestingly, these works reported discrepant results on lysophosphatidylcholine (LPC) content. Whereas Ståhlman and colleagues found increased LPC in HDL from dyslipidemic, diabetic subjects [
39], in Cardner’s work, it was reduced [
38]. This difference is of potential interest for the implications on the inflammatory properties of HDL. In fact, as it is well known, LPC is constituted by fatty acids that can be either anti-inflammatory (polyunsaturated fatty acids) or pro-inflammatory (arachidonic acid). In Ståhlman’s work, the observation that LPC is mainly associated with arachidonic acid suggests that small, dense HDLs may represent a biomarker of the inflammatory milieu in diabetes. Additionally, Ståhlman and colleagues have noted that atherogenic dyslipidemia, and not insulin resistance or hyperglycemia, is the main driver of HDL lipidome perturbations. In fact, the above described alterations were not observed in HDL isolated from normolipidemic, diabetic people [
39].
The reduction in sphingosine 1-phosphate (S1P) content of HDLs has been observed in diabetic patients [
40], with negative implications on HDL vasodilator activities.
2.4. Modifications of the Protein Component
Most studies agree with the observation that the levels of ApoA-I, the most prominent protein of HDL, are significantly reduced in T2DM. Multiple mechanisms are likely to account for this modification: (i) the affinity of ApoA-I for the small HDL particles typical of T2DM is reduced, leading to the dissociation of ApoA-I and the consequent accelerated clearance by the kidneys; (ii) the synthesis of ApoA-I may be reduced through a mechanism of inhibition of transcription factors driven by high glucose levels; (iii) the ApoA-I expression is reduced as a consequence of the insulin resistance [
41]; (iv) the binding of the pro-inflammatory protein serum amyloid A (SAA) to HDL is accompanied by the removal of ApoA-I [
37].
In general, the content of proteins in HDL is significantly altered in T2DM, as comprehensively illustrated in a recent study assessing the proteome of HDL from diabetic and healthy subjects. This work revealed that 17 proteins were increased and 44 were decreased in the disease status. Among the former, it is worth mentioning SAA, fibrinogen, ApoCII, and ApoCIII. Among the latter, relevant examples were ApoA-IV, apolipoprotein E (ApoE), apolipoprotein M (ApoM), and paraoxonases (especially PON1) [
38] (). The reported increase in plasma levels of ApoC-II and ApoC-III is consistent with the elevated TGs detected in diabetic subjects [
42]. Since glucose induces ApoC-III transcription, a mechanism that links hyperglycemia and hypertriglyceridemia in patients with T2DM has been suggested [
43]. Indeed, a recent multi-ethnic study demonstrated an adverse association between increased plasma ApoC-III levels and the risk of diabetes, while HDLs lacking ApoC-III were associated with lower incidence. These observations may suggest that ApoC-III negatively impacts HDL functions, probably by disrupting glucose homeostasis and enhancing circulating triacylglycerol levels [
44]. Consistent results were observed when HDL from adolescents with T2DM were analyzed. In this young population the typical feature of atherogenic dyslipidemia is apparent, with a significant increase in plasma TGs and a decrease in HDLs, which are also shifted to smaller particles. Interestingly, a significant reduction in all proteins, including ApoA-I, ApoA-II, ApoE, ApoM, and PON1 was found [
45].
2.5. Modifications of HDL Due to Glycation and Oxidation
Diabetic patients are under the conditions of hyperglycemia and oxidative stress. It is well known that the long-term exposure of proteins and lipids to high levels of glucose leads to the non-enzymatic glycation of several macromolecules, of which the HDL is a primary example [
46]. Simultaneously, oxidative stress may modify HDLs through multiple mechanisms, as demonstrated by the increase of 127% of oxidation as measured by the TBARS assay [
47], or the nitration of ApoA-I [
48]. Thus, collectively, these processes affecting HDL composition and function may be termed “glycoxilation” [
16].
The first evidence of non-enzymatic glycation of HDL in vivo was published in 1985. Curtiss and Witztum verified glycation in plasma lipoproteins of diabetic individuals using an innovative immunochemical approach that employed monoclonal antibodies recognizing glycosylated residues on proteins. The results showed that diabetic patients presented a large array of glycosylated proteins, including ApoA-I, ApoA-II, ApoB, ApoC-I, ApoE and albumin, primarily in HDL. Of note, the relevance of a high extent of glycosylation in TG-rich lipoproteins of diabetic individuals was explained by the transfer of glycosylated apoproteins from HDLs [
49]. Although this study has been carried out in only three hyperlipidemic, diabetic individuals with poor glucose control, the increased extent of HDL glycation has been later confirmed in larger cohort of T2DM subjects, and it has been related to plasma levels of glucose [
50]. Recently, an elegant work of proteomics comparing plasmas from control and diabetic individuals revealed that the latter present significantly higher glycated ApoA-I, suggesting that this parameter could be considered as valuable diagnostic tool to assess the metabolic state of diabetic patients that experience a glyco-oxidation stress during the half-life of the protein [
51].
Moreover, an in vitro study by Hedrick and collaborators showed that HDLs incubated with high concentrations of glucose had a fourfold increase in glycation, especially on the protein component, including ApoA-I, paraoxonases, and malondialdehyde, an important marker of oxidative stress [
16].
The above described compositional changes that HDLs undergo in T2DM have a significant impact on the antiatherogenic functions of these lipoproteins. In this section, we examine the most relevant reports documenting alterations of the anti‑ inflammatory, antioxidant, vasodilator properties of HDL occurring in T2DM, as well as modifications of the capacity to promote cholesterol efflux, the first, limiting step of the reverse cholesterol transport (RCT) process.So, from a practical point of view, no intervention to increase HDL‑C plasma concentration or to change its function, to date, has proven effective in randomized clinical trials. Thus, HDL dysfunction and its reduced levels should be considered as causal elements in the severity of atherosclerotic disease in individuals with T2DM. HDL functionality tests are not yet systematized so that they can be used in clinical practice and are restricted to the research scenario. Nevertheless, new approaches to systematize these methodologies, such as the use of apo AI or HDL mimetics and the possibility of combined therapies that target both the HDL particle and the cellular apparatus responsible for their interaction, remain to be explored. For now, the extensive volume of knowledge accumulated over years about the interaction between HDL and T2DM paves the way for a pathophysiology of severe atherosclerotic disease in these patients. In the near future, it is possible that this set may bring new perspectives for the characterization of risk and intervention.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10112233