4. Discussion
Heavy metals are released by many industrial activities and may be residual in water after different forms of remediation. Filtering of solubilized HMs is extremely expensive. Membrane filtration technology is preferred for its simplicity, energy efficiency, and manufacturing scalability [
13]. However highly concentrated minerals such as calcium sulphate and carbonate may deposit on the membrane causing so called “scaling” [
14], leading to increased flow resistance, requirement for frequent cleaning, and replacement of membranes. To reduce scaling, more expensive membrane modifications are needed [
15].
Because of the relative high cost of membrane filtration, phytoremediation appears to be an eco- friendly and economic alternative to remove HMs and metalloids from polluted water. However, the success of phytoremediation depends upon the identification of high biomass, metal tolerant plants.
Another problem is the adaptability of plants to existing technologies and approaches that usually implies a relatively fast flux of polluted water. The concept of pollutant sequestration in the growing biomass may sometime not be applicable when the growing rate is not in line with the wastewater flux.
Aquatic mosses cultivated ex-situ can be packed to adapt filtering containers of the desired volume and shape. Some of them have extremely coherent and resistant thalli so that they do not release debris, representing also a valid mechanical filter for particles. At the same time, they can be a natural support for the growth of bacteria usually part of bio-filtration, replacing less eco- friendly artificial materials [
11].
To the best of our knowledge, this is the first assessment of HM phytofiltration capacity of live aquatic moss species among the most versatile and resistant, Taxiphyllum barbieri. It has no problematic requirements to grow and, as other similarly resistant species such as Vesicularia dubyana, can represent an organism adaptable to several filtering needs of modern technologies.
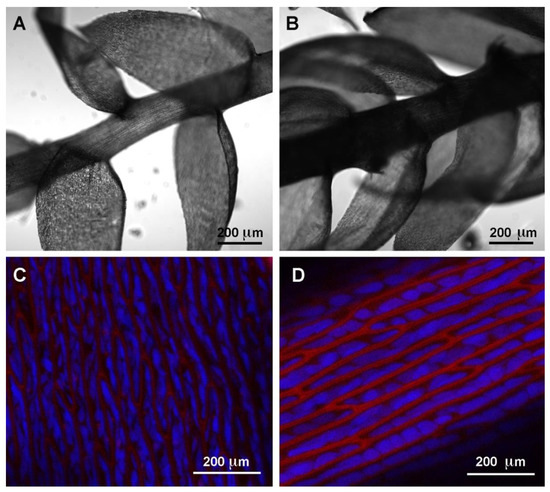
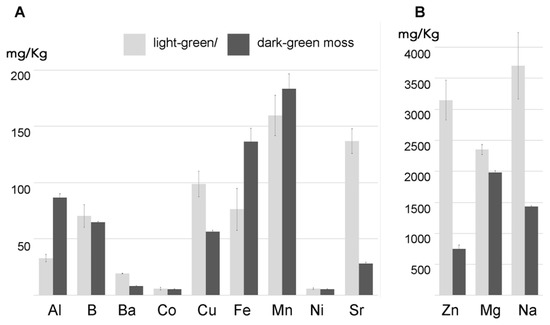
We tested the ability of two independently cultured mats of Taxiphyllum barbieri to capture some of the most dangerous HMs such as As, Cd, Pb, and Cr. We used two biomasses grown under different light conditions, showing different morphological characteristics, with the intent to answer the question of whether the same organism could be grown to better respond to different remediation needs. The morphological characteristics we monitored are shown in . They behave differently, accumulating various elements in a differentiated way. We show this in , by analyzing the accumulation of elements normally present in the culture medium used during the experiments.
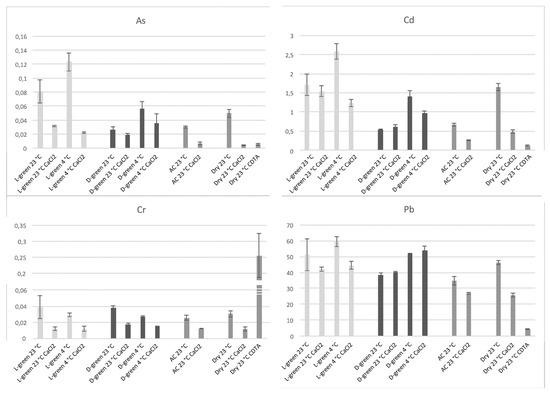
Since dry moss biomass was previously shown to be a valid biosorbent for Cd [
16], we evaluated if differently grown mosses accumulated elements because of interaction with pectins or because of other processes. We used washing with CaCl
2 to extract weakly bound elements or CDTA to remove pectins completely.
In live cells, metal uptake is considerably affected by variation in temperature due to the dependence on metabolism, even, biosorption, which is independent of the cell metabolism mechanism, is also affected because it involves sorption onto the cell surface based on physical and chemical interactions between metal and functional groups of the cell wall [
17]. As well evidenced in
Zooglea ramigera, the sorption of Cu(II) and Ni(II) ions is increased with decreasing temperature but the sorption of Pb(II), Cr(VI) and Fe(III) ions increased with increasing temperature [
18]. Bond rupture, caused by increasing temperature, enhances the number of active sites involved in metal sorption or the higher affinity of sites to metals has been ascribed to increased biosorption [
19]. Similarly, Kayalvizhi et al. (2014) reported that the biosorption capacity of an algal biomass, could be increased with an increase of temperature. The pores in the biomass become enlarged favoring the sorption process and diffusion of Cr(VI) ions [
20]. To explore the influence of this parameter, two different temperatures were used to incubate live moss: 23 and 4 °C.
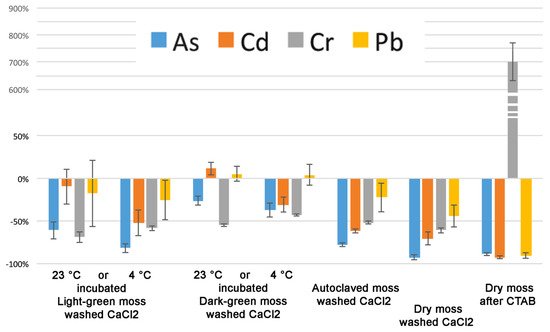
Our results, reported in show that despite the fact that most of the accumulation can be related to pectins, the differences between light- and dark-green moss are too important to depend entirely on the cell wall. Cr is evidently bound to a cellular fraction that is different from pectins. The CDTA extraction increased Cr concentration in the residual sample. Lead accumulation appeared to be related to uncharacterized cellular processes, different from ionic binding, but more efficient in dark-green moss. In general, as better evidenced by , the effect of washing treatments was different between light- and dark-green moss.
Reassured of the physiological difference of these differently cultivated biomasses we also analyzed the effect of zinc and copper co-accumulation on the accumulation of some more toxic pollutants. In fact, regardless of their toxicity or physiological role, the variations in uptake of some elements can influence the uptake and accumulation of other elements with synergistic or antagonistic effects.
A stimulating effect of Fe deficiency was observed in
N. caerulescens on Cd uptake and it was found to be connected with Fe-regulated transporter-like protein genes [
21]. Among the nutrient elements, Ca is related to Cd in
B. juncea and
S. alfredii [
22,
23]. Cd concentration in
N. caerulescens plants correlated also with Zn, Fe, and Cu concentrations [
24] and Zn uptake was reduced by Cu excess in soil [
25].
If aquatic mosses are to be used for water filtration and remediation, their behavior in the presence of multiple pollutants should be better investigated. In our study we can visualize that higher Cu concentration may positively correlate with increased Pb uptake by moss grown in optimal light. In general, our experimental setting did not allow to distinguish if element accumulation was due to uptake or adsorption but when differences correlate with treatments or gametophyte morphology, as shown in , a physiologic active contribution can be hypothesized and we may suggest an increased uptake under specific conditions.
The most interesting observation remains the different behavior of the two mats cultivated in different conditions. These developed diversified morphological aspects suggesting possible physiologic differences that need to be investigated further.
Several moss species have been found particularly efficient for HM accumulation [
7,
8] but in our work we suggest that each moss species could be cultivated differently so as to be adapted to the accumulation of different pollutants. Differences may be due to different accumulation capacity in the cell wall or intracellular compartments [
26] depending on physiologic adaptations.
In the experiments reported in and , the removal of HMs from the liquid medium was reported. As, Cd, and Pb removal was tested in a period as short as 6 h, up to 12 h. Arsenic (As(III)) was not removed from the liquid medium. Further experiments are needed to elucidate its behavior with both arsenite and arsenate uptake. The analysis of Cd and Pb removal in different periods of time, reported in , showed that even if the Pb total accumulation capacity in proportion to biomass is much higher than for Cd, the initial efficiency speed is similar.
We then proposed an experiment where Cd removal from liquid medium was analyzed at shorter time intervals (2 h), and compared to Cr(VI), it is another extremely important pollutant [
26]. Using a ten times larger biomass the HM removal appeared not to be influenced by saturation limitations and also showed how fast removal can be. A rapid wastewater phytofiltration appears to be an appealing possibility.
Phytofiltration capacity of an aquatic moss for application in polluted water remediation as a bio-filter, has only started to be investigated moving research to further its value as bioindicator [
6]. It was shown that the aquatic moss,
Warnstorfia fluitans took up As from water accumulating up to 4.5 mg of As per g of dry weight (DW) without influencing its biomass [
6]. The moss accumulated high levels of As similar to those found in As-hyperaccumulating fern,
Pteris vittata, which accumulated 3.5 mg As per g DW in its fronds without any sign of toxic effects [
6,
27].
A study found that another submerged aquatic plant species,
Vallinseria natans (Lour.) Hara, accumulated nearly 2 mg As per g DW when it was exposed to 5 mg/L arsenate for 7 days [
23]. Within a similar time period
W. fluitans had a higher As accumulation than
V. natans. It might be possible to use the term As “hyperaccumulator” here, as it refers to plants accumulating over 1 mg per g DW in their aboveground parts [
28,
29]. In aquatic moss, however, there is direct uptake via the thallus and HMs do not reach the green parts via root transport. The direct uptake by the whole plant body in submerged plants probably explains the higher concentrations found in them than in terrestrial and emergent plants [
30].
We need to consider that the studied moss plants here originated from a commercial source and were not optimized for phytoremediation. Plant clones could be further improved genetically through controlled growth under selective pressure to stimulate the up-regulation of resistance mechanisms [
31].
Sandhi and co-authors (2018) reported that the As concentration in
W. fluitans increased with increasing As concentration [
6], as found also in other studied species [
32]. The relationship between the internal and external concentrations was linear, at least up to the 100 mM arsenate (As(V)) treatment: in our case we used arsenite (As(III)) and this is the possible reason why the uptake was irrelevant. Indeed it is normal to have lower uptake of As(III) than As(V) in emergent and terrestrial plants [
30]. Anyhow the opposite could also be observed in submerged plants, for example in
Spartina spp. [
33] As uptake was higher from arsenite than from arsenate-bearing solution. At least we evidenced here the tolerance of moss to As treatment. Further studies are certainly required to establish the potential As(V) uptake.
To summarize, this work demonstrated that T. barbieri would be a suitable candidate for the phytofiltration of Pb (>100 g/kg DW in 6 h), Cd (about 4.3 g/kg DW in 6 h) and Cr (about 19.4 g/kg DW in 6 h) from contaminated water since it has high and rapid accumulation capacity for these HMs. A comprehensive comparison with other technological methods should include a long term environmental impact but this is too difficult to obtain. Nonetheless the comparison with an equivalent mass of synthetic nylon, provided here an interesting indication.
The high removal rate from the water of some HMs has to be coupled with the easy cultivation and the strong thallus that can work as support for nitrifying bacteria. This makes
T. barbieri a very good candidate for phytoremediation/phytofiltration of water polluted by specific human activities
[1] or for complete water remediation downstream from other purification processes offering innovative and more eco-compatible solutions.