The unique mechanism of CAR-T therapy in addition to the high positive results mentioned above also triggered wide development programmes assessing this novel therapy in different haematologic malignancies as well as solid tumours.
4.1. CAR-T Therapy in AML
In 2020, the American Cancer Society estimated AML to represent 1% of all cancers. It is mainly a disease of the older population, being uncommon before the age of 45, with the average age at first presentation of 58 years. Its incidence is slightly more common among men than women. It is the most common leukaemia in adults and the second most common leukaemia in children [
30,
31].
Currently, with the standard 7 + 3 regimen (consisting of cytarabine for 7 days and anthracycline for 3 days), a CR rate of up to 80% has been achieved in young adults and up to 60% in older adults who are 60 years of age and above. This is followed by post-remission induction, which differs according to different factors, e.g., patient’s age, general condition, molecular prognostic stratification, etc. [
32,
33]. However, patients who do not achieve remission from the first-line regimens and those who relapse pose a serious problem for the haematologists with the continuous need for the development of effective therapies [
34].
In ALL, the presence of a specific target such as CD19 supported the scientific concept behind the development of a specific targeted CAR-T therapy. However, the lack of a specific cell target for AML makes it difficult to treat this disease with targeted immunotherapy [
35,
36]. Currently, different researchers are attempting to identify an antigen or a group of antigens that are predominantly expressed on the myeloblast and not the normal tissues. This concept of specifically targeting surface antigens on myeloblasts is important to be able to target AML disease while avoiding the exposure of normal tissues to unnecessary immunotherapy, which could result in toxicity [
37].
The concept of using targeted immunotherapy for patients with AML was successfully applied by treating CD33-positive AML patients (adults and children over 2 years of age) with chemotherapy and the CD33-targeted antibody-drug conjugate, gemtuzumab ozogamicin [
38,
39]. The positive results of this combination led to the approval of this combination in adults and children over 2 years of age who suffer from CD33-positive AML [
40,
41].
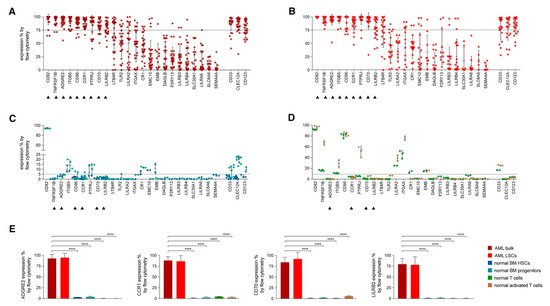
In an attempt to identify the ideal candidates for CAR-T therapy in AML, Perna et al. conducted an extensive analysis of large datasets of transcriptomics and proteomics from malignant and normal tissues [
42] (). The researchers performed surface-specific proteomic studies in a diverse panel of AML cell lines (THP1, Mono-mac, Kasumi, Molm13, OCI/AML3, and TF-1). The team performed a mass spectrometric analysis, which led to the identification of 4942 proteins. They enriched their protein sets by adding the findings from other similar studies conducted on other AML cell lines (e.g., NB4, HL60, THP1, PLB985, CD32, CD33, CE 96, CD99, and K562). The researchers used data from the Human Protein Atlas, the Human Proteome Map, and the Proteomics Database to identify the protein expression information in different normal tissues/organs such as liver, gallbladder, pancreas, stomach, gut, rectum, testis, epididymis, prostate, breast, etc. [
43,
44,
45]. Consequently, they looked for CAR-T therapy targets that are overexpressed in AML versus normal tissue, and they were able to identify 24 molecules that fulfil this criterion (A,B). Using flow cytometry to analyse the expression of the 24 candidates in AML specimens, they managed to detect nine targets that are detected in >75% of the cells (expression range: 78–99%, mean 82%): CD82, TNFRSF1B (also known as CD120b), ADGRE2 (also known as EMR2 or CD312), ITGB5, CCR1 (also known as CD191), CD96, PTPRJ (also known as CD148), CD70, and LILRB2 (also known as CD85d). Six of the nine targets were expressed in <5% in normal tissues (with ADGRE2, CCR1, CD70, and LILRB2 expressed in <5% in freshly purified and activated T cells from healthy donors), whereas the other three were expressed in the normal tissues at a slightly higher level with the maximum expression of 20% (C,D). Still, all were much less expressed than their corresponding expression in the primary AML cells. (E) [
42].
Figure 3. Flow Cytometric Analyses in Primary AML Samples and Normal Haematopoietic Cells. (
A–
D) Expression (% positive) of the 24 candidate antigens and the three CAR targets in current clinical investigation (most right three) in bulk AML population (
A), in leukaemic CD34
+CD38
− cells (
B), in normal BM CD34
+CD38
− CD45RA
− CD90
+ HSCs (blue), CD34
+ CD38
+ progenitor cells (light blue) (
C), or in freshly purified (green) or activated (brown) normal CD3
+ peripheral blood T cells (
D). Data are represented as mean ± SD. (
E) Summary of expression levels (mean ± SEM) of four top targets in indicated cell populations. ****
p < 0.0001 (Student’s
t-test). Reprinted from Perna F, Berman SH, Soni RK, et al. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell 2017; 32: 506–519.e505 with permission from Elsevier [
42].
Many pre-clinical studies were also conducted to assess the different possible targets for CAR-T therapy in AML populations, which led to the start of the Phase I clinical studies. These studies tested a wide range of ages, from as young as 6 months to up to 90 years of age. The number of patients treated in these studies was small. Cummins et al. treated six patients with CAR-T therapy. Two patients achieved complete remission (CR), three patients achieved partial remission (PR), and one patient had progressive diseases (PD) [
46]. Richie et al. also treated four patients with CAR-T, with all of them initially achieving PR or stable disease (SD), then they relapsed [
47]. Of note, both Cummins et al. and Richie et al. used autologous T-cell sources. Numerous studies are ongoing, with data still to be presented [
48,
49].
4.2. CAR-T Therapy in CLL
In order to assess if CAR-T therapy can target CLL cells, a group of researchers from the NCI in Bethesda managed to construct two CARs. They subsequently chose the one that has shown the best anti-tumour activity in vitro for further testing in CLL clinical trials [
50].
One of the earliest observations of clinical activity in CLL was reported by Porter et al. in 2011 [
51]. A patient who had R/R CLL was infused with a low dose (approximately 1.5 × 10
5 cells per kilogram) of autologous CAR-T cells. A real-time polymerase chain reaction detected DNA encoding anti-CD19 CAR-T after 1 day of the infusion of the cells. The infused CAR-T cells expanded to a level more than 1000 times the initial engraftment. The patient achieved CR. The toxicity profile was as expected, with the only grade 3/4 adverse event related to CAR-T therapy being lymphopenia as well as hypogammaglobulinemia, which was a chronic effect. The patient also suffered from tumour lysis syndrome. Leukaemia, as well as normal B cells-expression CD19, disappeared from the patient’s blood and bone marrow cells. A high level of CAR-T cells was maintained in the patient’s blood and bone marrow for 6 months, and the patient’s remission was ongoing at 10 months post-engraftment [
52].
Following that, CAR-T therapy was investigated in different clinical trials, with more than 130 patients being tested in the trials. Most of these patients were heavily pre-treated, some relapsed post haematopoietic cell transplant [
53,
54]. Others were cases treated as they progressed to Richter syndrome. Despite the heterogeneity of the patient population and the adverse prognostic factors, a CR rate in the range of 20–30% of the patients with estimated progression-free survival (PFS) at 18 months of 25% was achieved. These studies have shown the potential activity of CAR-T therapy in the CLL patient population [
55,
56].
Ibrutinib, which is a breakthrough treatment for patients with CLL, was found to improve response in clinical trials. In one of the trials, treatment with CAR-T therapy following ibrutinib in three patients resulted in responses in all three patients, with one achieving CR [
57]. Two other studies combining ibrutinib and CAR-T therapy have shown very promising response rates of 80% in two series of 19 patients with an MRD eradication in the bone marrow of around 90% among responders. The safety profile was not different from that of other patients with other indications when treated with CAR-T therapy, with the CRS being the main concern [
58,
59]. Other studies are ongoing to explore the full potential of CAR-T therapy in patients with CLL [
60].
4.3. CAR-T Therapy in Hodgkin Lymphoma (HL)
Data from two studies testing CAR-T therapy targeting the CD30 antigen in patients with HL were recently presented in the Transplantation and Cellular Therapy Meetings of American Society for Blood and Marrow Transplantation (ASBMT) and Center for International Blood & Marrow Transplant Research (CIBMTR) and highlight the potential role of anti-CD30 CAR-T cells for this disease [
61,
62]. In one of the trials, the investigators treated the patients with a lymphodepletion chemotherapy that included cyclophosphamide and fludarabine (FC) before CAR-T cell infusion. With 14 patients enrolled in this Phase I study, the median age was 30 years (range: 17–69 years). Patients were CD30 positive HL and heavily pre-treated with a median of five prior regimens. Most patients previously received a checkpoint inhibitor and the monoclonal antibody brentuximab targeting CD30. Patients received a single infusion of one of three dose levels: 2 × 10
7 cells/m
2, 1 × 10
8 cells/m
2, or 2 × 10
8 cells/m
2. The investigators found the expansion and persistence of the CAR-T cells to be dose-dependent. Of the 14 patients treated, important safety findings were seen in four patients who developed grade 1 CRS, and some patients developed maculopapular rashes that disappeared without any treatment. Other adverse events were in the form of alopecia, gastrointestinal toxicities, and transient cytopenias. At 6 weeks, 12 patients were evaluable for response, seven patients achieved CR, and one patient achieved PR. One of the CR patients showed the response by 6 weeks and maintained the remission for more than a year [
61].
In another Phase I/II study, patients received one of two dose levels in a standard 3 + 3 design. The doses tested in Phase I were 1 × 10
8 cells/m
2 or 2 × 10
8 cells/m
2, and the Phase II part of the study tested the selected Phase I dose in more patients [
62]. In both Phases of the study, a total of 29 patients were enrolled with a median age of 35 years (range: 23–69 years). All patients had a refractory disease and were heavily pre-treated with a median of eight previous regimens. There were 28 evaluable patients, of which 26 (8 in Phase I and 18 in Phase II) received CAR-T cell infusion (24 patients had classic HL, and the other two had T-cell lymphomas). In the eight patients enrolled in the Phase I part of the study, three patients received the lower dose of CAR-T cells (1 × 10
8 cells/m
2) and all progressed. Of the other five patients who received the high dose (2 × 10
8 cells/m
2), three patients had CR, one patient had SD, and one patient had PD. As there were no dose-limiting toxicities, the 2 × 10
8 cells/m
2 was selected for the Phase II part of the study. Bendamustine (B) single agent was given for lymphodepletion in Phase I of the study. However, as response and CAR-T cell expansion were suboptimal, the investigators decided to add fludarabine (F), i.e., bendamustine and fludarabine combination (BF).
Out of the 18 patients treated in Phase II, four developed CRS (three grade 1 that resolved spontaneously, and one was grade 2 that was managed by tocilizumab). There were also nine patients who had mild rashes after the infusion. Patients also had cytopenias in the form of grade 3 or high neutropenia in 3 patients (12%), thrombocytopenia and lymphopenia each occurring in four patients (15%). Of the 18 patients who received CAR-T cell infusion, 14 patients achieved CR (78%), of which two had a response longer than 1 year. Another two patients (11%) achieved PR; one patient had SD (5%). Only one patient had PD (6%). Of note, the CR rate was higher in the patients who had the combination of BF (78%) versus the patients who received B monotherapy (37%), with survival also being longer in those who had BF than those who had F alone (median of 389 days vs. 55 days). After a median follow-up of 108 days (range not reported), progression-free survival was 164 days for 19 evaluable patients who had active disease at the time of lymphodepletion. Patients who received the higher dose of CAR-T cells with the combination lymphodepletion regimen appeared to have a longer survival than those treated with a lower dose and single-agent bendamustine (median = 389 days vs. 55 days;
p = 0.0004) [
62].
These data suggest the potential role of CAR-T therapy targeting CD30 HL. However, further studies with larger numbers of patients and longer follow-up periods are needed to identify the role of anti-CD30 CAR-T cell infusion in HL.
4.4. CAR-T Therapy in MM
Multiple myeloma (MM) is a plasma cell cancer, and despite the recent advances in treatment and the use of immunomodulatory, proteosome inhibitors, and other novel therapies, there is still no cure for it [
63,
64]. Current available therapies improve disease outcomes, but patients will ultimately relapse. Each relapse negatively affects survival chances for the patients [
65,
66].
Superior results are seen with CAR-T therapy in non-Hodgkin lymphoma and ALL encouraged the testing of CAR-T cell therapy in the disease of MM. B-cell maturation antigen (BCMA) is a protein and a tumour necrosis factor that is expressed by normal and malignant plasma cells including MM cells [
67,
68]. A specific CAR-T cell therapy (bb2121) was produced by the transduction of autologous T cells with a lentiviral vector that encodes a second-generation CAR-T that incorporates an anti-BCMA single-chain fragment, a CD137 (4–1BB) costimulatory motif, and a CD3-zeta signalling domain [
69,
70].
CAR-T therapy has shown the potential to benefit patients with MM. In a pre-clinical Phase I study conducted by Raje et al., bb2121 (a CAR-T agent that targets BCMA) showed promising activity in patients with R/R MM [
71]. The study consisted of two phases: dose escalation and dose expansion. In the dose escalation phase, a single infusion of CAR-T cells was tested in groups of patients in a dose-escalating manner. The doses that were tested were 50 × 10
6, 150 × 10
6, 450 × 10
6, and 800 × 10
6. In the dose expansion phase, only two doses were tested: 150 × 10
6 and 450 × 10
6. The study enrolled heavily pre-treated patients who received at least three previous lines of therapy (the median was 7 and the range was 3–14). With the exception of one patient, all patients previously failed an autologous stem cell transplant. All patients previously received and relapsed or were refractory to an immunomodulatory agent and a proteasome inhibitor. Some patients also had extramedullary disease (27%), and 45% of the patients had a high-risk cytogenetic profile with the presence of t(4;14), t(14;16), or del(17p). Safety was the primary endpoint of the study, and the safety results of 33 evaluable patients (out of 36 enrolled in the study) were reported. The main toxicity was haematologic toxicity, with events of grade 3 or higher reported for 85% of the patients in the form of neutropenia. Other grade 3 or higher toxicities were reported in the form of leukopenia, anaemia, and thrombocytopenia in 58%, 45%, and 45% of the patients, respectively. The non-haematologic toxicities were in the form of CRS, which occurred in 25 patients (76%). Most of the patients (23 patients, 70%) had a maximum of grade 2 CRS, and only two patients (6%) had grade 3 CRS. Neurologic toxicities occurred in 14 patients (42%), with only one patient suffering from a reversible grade 4 event and all the remaining patients having only grade 1 or 2. A CR was achieved in 15 patients (45%), one patient had PR (3%), and a median of PFS of 11.8 months was achieved. Of note, six patients out of the 15 patients ultimately relapsed. It is important to note that some responders had MRD negativity. The investigators concluded that BCMA-directed cellular immunotherapy for patients with R/R MM had toxicities similar to what was reported in other CAR-T cell therapies used for other indications. The therapy also resulted in promising responses in the form of CR/PR with doses of 150 × 10
6 in this heavily pre-treated patient population of R/R MM [
71].
In another first-in-human study (FIH), the anti-B-cell maturation antigen BiTE molecule was assessed in patients with R/R MM [
72]. Patients who relapsed after at least two prior lines of therapies and no extramedullary disease were treated in the study. Patients received up to 10 cycles of treatment, given as an infusion every 4 weeks. The length of the cycle was 6 weeks. MRD was assessed using flow cytometry, and MRD negativity was defined as the presence of <1 cell/10
4 bone marrow cells. A total of 42 patients were enrolled in the study. The median age of the patients was 65 years. Patients had a median duration of MM of 5.2 years. The median number of cycles received by the patients was 1 (range: 1–10 cycles), while the responders received a median of 7 cycles. The doses received by the patients were in the range of 0.2–800 µg/d. Reasons for treatment discontinuation were disease progression in 25 patients (60%), adverse events in seven patients (17%), and death in four patients (10%). Likewise, three patients (7%) withdrew from treatment as they received the maximum allowable cycles in the study (10 cycles), one patient (2%) withdrew consent for continuation of treatment, and two patients (5%) were still on treatment at the time of the presentation of these data. Safety results have shown that the 800 µg/d was not tolerated, with two out of the three patients enrolled having a DLT, with one having a grade 3 CRS and the other one suffering from neurotoxicity (polyneuropathy) that started as grade 2 and progressed to grade 3. Both events were later resolved. For the patients who received the 400 µg/d dose, one patient had grade 1 polyneuropathy that progressed to grade 3 but ultimately resolved by week 12. CRS was seen in 16 out of the 42 patients (38%); however, it was treated with glucocorticoids, antihistamines, antipyretics, and analgesics, with one patient receiving tocilizumab for grade 2 CRS. Additionally, there were five patients who had increases in liver enzymes (ALT and/or AST > 3× times the upper limit of normal). Among them, four patients received the 400 µg/d. However, one of the four patients who had increased liver enzymes had increased enzymes at baseline. At the 400 µg/d dose, which was received in 10 patients, seven achieved a response rate of 70%. Among the seven responders, there were five patients who achieved MRD negative CRS. For the remaining two patients, one had a PR, and one had a very good PR. All the responses occurred in the first cycle, and some responses lasted >1 year. The investigators concluded that these results are promising and warrant further investigation [
72].
The CARTITUDE-1 was a Phase Ib/II trial that enrolled adult patients who were heavily pre-treated with three or more prior lines of therapy or were double-refractory to an immunomodulatory drug and a proteasome inhibitor. Patients received a single low dose of ciltacabtagene autoleucel (cilta-cel), an autologous CAR-T therapy. Cilta-cel is a bioengineered T-cell receptor construct with a CD3ζ signalling domain, a 4-1BB costimulatory domain, and two BCMA binding domains. The Phase I study objectives were safety and defining a dose to carry forward to the Phase II part of the study. Patients underwent apheresis and cell collection to produce the CAR-T cells. The patients had lymphodepletion with 3 days of fludarabine 30 mg/m
2 plus cyclophosphamide 300 mg/m
2. The dose received of cilta-cel was a single dose of 0.75 × 10
6 cells/kg (range: 0.5–1.0 × 10
6 cells/kg). At the time of the cut-off level of 1 September 2020, 113 patients were enrolled, with 97 receiving CAR-T (Phase Ib 29 patients and Phase II 68 patients). The median age of the patients was 61 years (range: 43–78 years), and the median number of prior lines of therapies was 6 (range: 3–18). Haematologic adverse events were the most common, with neutropenia, anaemia, and thrombocytopenia occurring in 96%, 81%, and 79% of the patients, respectively. Most haematologic adverse events resolved quickly without complications. Non-haematologic adverse events were CRS (95% of patients) and neurotoxicity (21% of patients). Most CRS events were grade 1/2 (95%). All CRS events resolved within 14 days of their onset in all but one patient. Only 10% of the patients who suffered from neurotoxicity had their events as grade ≥3. The number of deaths was 14, five of which were due to PD, three due to adverse events unrelated to the CAR-T therapy, and six due to adverse events related to the therapy. The efficacy results have shown that 67% of the patients achieved stringent CR, 26% achieved a very good PR, and 4% had PR. There were 57 patients who were evaluable for the assessment of MRD, 93% of whom became MRD negative. PFS at 12 months was 77%. The investigators recommended the testing of this CAR-T therapy in a larger clinical trial [
73].
The efficacy and safety of Ide-cel (bb2121) in patients with relapsed and refractory multiple myeloma and in subjects with high-risk multiple myeloma (KarMMA Phase II study) also reported the results of Ide-cel, another CAR-T therapy targeting BCMA. Based on the Phase I promising activity, the Phase II study enrolled 140 heavily pre-treated patients with MM. The median age of the patients was 61 years. For lymphodepletion, the patients received cyclophosphamide 300 mg/m
2 and fludarabine 30 mg/m
2 for 3 days. This was followed by the infusion of CAR-T cells in the range of 150–450 × 10
6. Of the 128 heavily pre-treated patients (median of prior 6 regimens) who received Ide-cel, and with a median follow-up period of 11.3 months, the overall response rate ORR (the primary endpoint of the study) was 73%, and the PFS was 8.6 months. The most common adverse events of any grade were cytopenias and CRS occurring in 97% and 84% of the patients, respectively. CRS was mainly grade 1/2, with only 5% having grade 3, one patient having grade 4, and one patient dying of CRS at the 300 × 10
6 dose of CAR-T. The response was durable, with 36% having CAR-T cells detected at 12 months. The investigators concluded that Ide-cel has demonstrated deep and durable responses in this heavily pre-treated patient population with an acceptable safety profile [
74].