Alphaviruses are mosquito-borne pathogens distributed worldwide in tropical and temperate areas causing a wide range of symptoms ranging from inflammatory arthritis-like manifestations to the induction of encephalitis in humans. Historically, large outbreaks in susceptible populations have been recorded followed by the development of protective long-lasting antibody responses suggesting a potential advantageous role for a vaccine.
- alphavirus
- antibody
- immunity
- alphavirus vaccine
1. Introduction
2. Antibody-Mediated Alphavirus Immunity
2.1. Virus-Specific Antibody Kinetics Upon Natural Infection with Alphaviruses
2.2. Experimental Evidence of the Role of Antibodies in Alphavirus Immunity
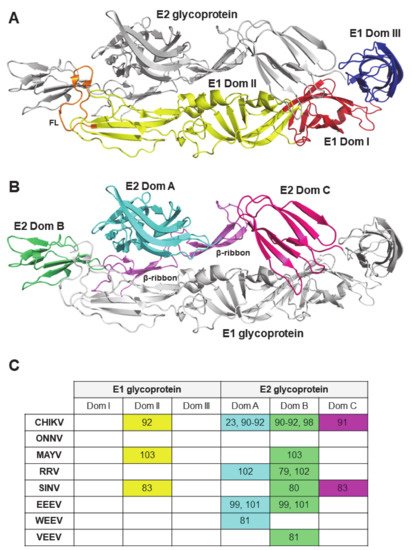
2.3. Viral Antigenic Regions Targeted by Neutralizing Antibodies
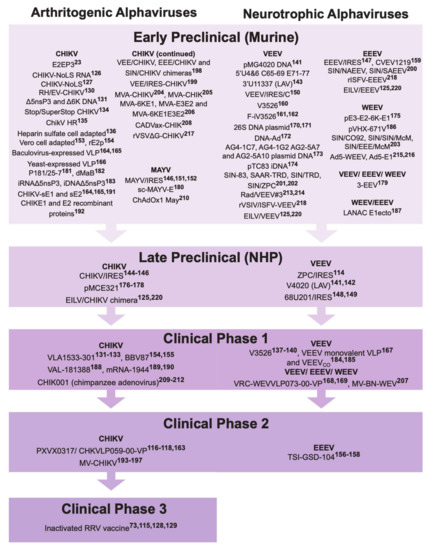
3. Alphavirus Vaccine Development
|
Vaccine Against Virus |
Name |
Strain Vaccine Modelled After |
Phase |
Immunization |
Challenge |
Humoral Immune Response(s) |
Ref |
|||
|---|---|---|---|---|---|---|---|---|---|---|
|
Dose |
Route |
Schedule |
Dose (Strain, Genotype) |
Route |
||||||
|
Live-attenuated |
||||||||||
|
CHIKV, ONNV |
RH-CHIKV EV-CHIKV RHEV-CHIKV |
LR2006 OPY1 |
C57BL/6 mice, 3 week old |
106 PFU |
s.c. in the ventral side of the right hind footpad |
Single dose |
106 PFU LR2006 OPY1 or WT-ONNV IMTSSA/5163, 3 mpim |
s.c. in the ventral side of the right hind footpad |
IC50, 613 (RH-CHIKV), 3407 (EV-CHIKV), 921 (RHEV-CHIKV) |
[130] |
|
CHIKV |
Δ5nsP3 (VLA1553-301 in clinical trials) and Δ6K |
LR2006 OPY1 |
C57BL/6 mice, 5 to 6 week old |
104 or 105 PFU |
s.c. in both flanks |
Single dose |
106 PFU LR2006 OPY1, 7 wpim |
s.c. |
NT50, 100 to 1000 |
|
|
Cynomolgus macaques, 3–4 years old |
105 PFU |
s.c. in the right upper back side |
Single dose |
100 AID50 (corresponding to 7000–10,000 PFU) LR2006 OPY1, 123 dpim |
i.v. |
NT50, >1000 |
||||
|
Human clinical trial, Phase 1 |
3.2 × 103, 3.2 × 104 or 3.2 × 105 TCID50 |
i.m. |
Two doses (0 and 6 months, or 0 and 12 months) |
NA |
NA |
GMT, 592.6 to 686.9 |
||||
|
CHIKV |
CHIKV-NoLS |
LR2006 OPY1 |
C57BL/6 mice, 21 days of age |
104 PFU |
s.c. |
Single dose |
104 PFU of LR2006 OPY1 or Ross River virus, 30 dpim |
s.c. |
<10% cells infected at 10-1 serum dilution |
[127] |
|
CHIKV |
Stop CHIKV SuperStop CHIKV |
LR2006 OPY1 |
C57BL/6 mice, 5 week old |
104 PFU |
s.c. |
Single dose |
ND |
ND |
~5–25 (Stop CHIKV) and ~10–25 (SuperStop CHIKV) fold reduction compared to mock |
[134] |
|
CHIKV |
ChikV HR |
37997 |
C57BL/6 mice, 28 days of age |
∼103 PFU |
s.c. into the left footpad |
Single dose |
103 PFU CHIKV SL15649, 28 dpim |
s.c. in the footpad |
PRNT50, 5 to ~500 |
[135] |
|
CHIKV |
Heparin sulfate cell culture adapted |
LR2006 OPY1 |
CD-1 mice, 21 days old |
105 GE |
s.c. in the rear footpad |
Single dose |
103 PFU LR2006 OPY1, 21 dpim |
NA |
~40 to 1000 fold change compared to mock |
[136] |
|
VEEV |
V3526 |
IA/B Trinidad donkey |
BALB/c, 6 to 8 week oldC3H/HeN mice, 6 to 8 week old |
105 PFU |
s.c. |
Single dose |
105 PFU of TrD, 28 dpim |
NP |
ND |
|
|
Cynomolgus macaques (age not specified) |
2.5 × 106 PFU |
s.c. |
Single dose |
∼108 PFU VEEV IE 68U201, 8 wpim |
aerosol |
PRNT80, 28 to 2560 |
||||
|
Rhesus macaques (2 to 4 years old) |
1.3 × 105 or 7.5 × 104 PFU |
s.c. or i.t./i.s. |
Single dose |
ND |
ND |
PRNT80, ~80 to 300 |
||||
|
Human clinical trial, Phase 1 |
25 or 125 PFU |
s.c. |
Single dose |
NA |
NA |
NA |
||||
|
VEEV |
V4020 |
IA/B Trinidad donkey |
BALB/c mice, 4 to 8 week old |
104 PFU |
s.c. |
Single dose |
104 PFU of VEEV TrD, 28 dpim |
s.c. |
PRNT80, 160 to1280 |
|
|
Cynomolgus macaques (age not specified) |
~104 PFU |
s.c. in the right leg |
Single dose (or second dose at 2 x 104 PFU i.m. if did not seroconvert) |
106 to 107 PFU of the VEEV TrD, 73 dpim |
aerosol |
PRNT80, >640 |
||||
|
EEEV |
5′U4&6 C65-69 E71-77 3′U11337 mutants |
FL93-939 |
CD-1 mice, 5 to 6 week old |
1.5 × 105 GE |
s.c. in footpad, or i.c. |
Single dose |
105 PFU EEEV FL93, 21 dpim |
s.c. in both footpads |
PRNT80, 16 to ~4000 |
[143] |
|
Live-attenuated (IRES) |
||||||||||
|
CHIKV |
CHIKV/IRES |
LR2006 OPY1 |
A129 mice, 3 or 10 week old |
104 PFU |
i.d. |
Single dose |
100 PFU LR2006 OPY1, 94 dpim |
i.d. |
PRNT80, >320 |
|
|
C57BL/6 mice, 3 week old |
105 PFU |
s.c. in the hind leg |
Single dose |
106.5 PFU Ross CHIKV, 21 dpim |
i.n. |
Mean PRNT80, 62 |
||||
|
A129 mice, 8 to 10 week old |
105 TCID50 |
s.c. |
Single dose |
100 PFU LR2006 OPY1, 50 dpim |
i.d. |
Mean PRNT80, 1152 |
||||
|
Cynomolgus macaques, >3 years old |
105 PFU |
s.c. or i.d. |
Single dose |
105 PFU LR2006 OPY1, 52 dpim |
s.c. in the upper deltoid |
PRNT80, 40 to 640PRNT50, 160 to 1280 |
||||
|
ONNV |
CHIKV/IRES |
LR2006 OPY1 |
A129 mice, 6 to 7 week old |
104 PFU |
i.d. |
Single dose |
105 PFU ONNV SG650, 38 dpim |
i.d. |
PRNT80, 160 |
[146] |
|
VEEV |
ZPC/IRESv1, ZPC/IRESv2 |
ID ZPC738 |
CD-1 mice, 6 to 8 week old |
105 PFU |
s.c. in the scruff of the back |
Single dose |
105 PFU VEEV 3908, 4 wpim |
s.c. or aerosol |
PRNT80, 40 to 324 |
[114] |
|
Cynomologous macaques, age not specified |
105 PFU |
s.c. in the upper deltoid |
Single dose |
~ 8 × 105 to 9 × 106 PFU VEEV 3908, 35 dpim |
aerosol |
PRNT80, <20 to 20PRNT50, <20 to 160 |
||||
|
EEEV |
EEE/IRES |
FL93-939 |
NIH Swiss mice, 3 to 4 week old |
104 PFU |
s.c. in the medial thigh |
Single dose |
103 PFU of FL93-939, 4 wpim |
i.p. |
PRNT80, 160 to 640 |
[147] |
|
VEEV |
68U201/IRESv1 68U201/IRESv2 |
IE 68U201 |
CD1 mice, 6 to 8 week old |
105 PFU |
s.c. in right hind leg |
Single dose |
(Lethal dose, NP) 68U201 at 1, 3, or 12 mpim |
s.c. |
PRNT80, 64 to ~300 |
|
|
Cynomolgus macaques (age not specified) |
105 PFU |
s.c. in the upper deltoid |
Single dose |
4 × 104 PFU VEEV IE 68U201, 49 dpim |
aerosol |
PRNT80, ~100 to 340 |
||||
|
VEEV |
VEEV/IRES/C |
IA/B Trinidad donkey |
CD-1 mice, 8 week old |
105 PFU |
s.c. |
Single dose |
104 PFU of VEEV 3908, 6 wpim |
s.c. |
Mean PRNT80, 184 |
[150] |
|
MAYV |
MAYV/IRES |
MAYV-CH |
BALB/c, 6 week old |
2 × 105 PFU |
s.c. i.pl. route |
Single dose |
2 × 105 PFU of WT MAYV, 28 dpim |
s.c. i.pl. route |
PRNT50, >640 (at 21dpi) |
|
|
AG129 |
2 × 104, 2 × 103 or 2 × 102 PFU |
s.c. i.pl. route |
Single dose |
2 × 103 PFU of WT MAYV, 14 dpim |
s.c. i.pl. route |
ND |
||||
|
CD-1, 28-day old |
105 PFU |
s.c. over the dorsum |
Single dose |
ND |
ND |
PRNT80, 160 to ≥ 640 |
||||
|
AG129, 5 to 8 week old |
104 PFU |
i.d. on the left foot |
Single dose |
104 PFU of WT MAYV, 29 dpim |
s.c. |
PRNT80, 320 to ≥ 640 |
||||
|
Inactivated |
||||||||||
|
CHIKV |
Vero cell adapted |
DRDE-06 |
Swiss albino mice, 3 to 4 week old |
10, 25 or 50 ug |
s.c. |
Three doses (0, 14 and 28 days) |
ND |
ND |
PRNT90, 6400 |
[153] |
|
CHIKV |
BPL/formalin-inactivated CHIKV BBV87 (in clinical trials) |
IND-06-AP3 |
BALB/c mice, 4 to 6 week old |
10, 20 or 50 μg |
i.m. |
Two doses (0 and 14 days) |
2.5 x 104 TCID50 IND-06-AP3, 4 or 22 wpim |
i.n. |
GMT, NT50, 80 to 1280 |
[154] |
|
Human clinical trial, Phase 1 |
10, 20 or 30 μg |
i.m. |
Three doses (0, 29 and 57 days) |
NA |
NA |
NA |
[155] |
|||
|
RRV |
Vero cell culture-derived whole-virus RRV vaccine Ross River Virus (RRV) Vaccine |
T48 |
CD-1 mice, 7 to 8 week old |
0.0025, 0.01, 0.039, 0.156, 0.625, 2.5 or 10 μg |
s.c. |
Two doses (0 and 28 days) |
106 TCID50 RRV T48, 42 dpim |
i.v. |
Mean NT, ≤2.9 to 46.2 |
|
|
A129 mice, 7 to 8 week old |
0.063, 0.25 or 1 μg |
i.m. |
Two doses (0 and 21 days) |
102.5 TCID50 T48, 42 dpim |
s.c. into left footpad |
Mean NT, ≤14 to 21 |
||||
|
CD-1 mice, age not specified |
10 μg |
s.c. |
Two doses (0 and 28 days) |
106 TCID50 T48, 6 wpim |
i.v. |
1000 TCID50 |
||||
|
Guinea pigs (Duncan Hartley), age not specified |
10 μg |
s.c. |
Single or two doses (0 and 6 weeks) |
106 TCID50 T48, 10 or 34 wpim |
i.v. |
NP |
||||
|
Human clinical trial, Phase 1/2 |
1.25, 2.5, 5, or 10 μg |
i.m. |
Three doses in escalation (0, 21 days, 6 months) |
NA |
NA |
GMT, 50 to 520.9 |
||||
|
Human clinical trial, Phase 3 |
2.5 ug |
i.m. |
Three doses (0, 3 weeks, 6 months) |
NA |
NA |
μNT GMT, ~0 to 85 |
||||
|
EEEV |
TSI-GSD-104 (formalin inactivated) |
PE-6 |
Human clinical trial, Phase 2 |
NP |
s.c. (0 and 28 days), i.d. (6 months) |
Three doses (0, 28 days and 6 months) |
NA |
NA |
PRNT80 >40 in 60% subjects (primary doses) versus 84% subjects (completed the 2-dose primary series and the 6-month dose) |
|
|
EEEV |
fCVEV1219 iCVEV1219 gCVEV1219 |
CVEV1219 |
BALB/c mice, 6 to 8 week old |
0.1 to 5 µg of inactivated EEEV |
i.n., s.c. or i.m. |
Single dose or two doses (0 and 28 days) |
Lethal dose of EEEV FL93-939, at 28 dpim (single dose) or 56 dpim (two doses) |
aerosol |
PRNT80, ~1 to 1000 |
[159] |
|
VEEV |
V3526 virus |
V3526 |
BALB/c mice, 6 week old |
0.2 μg (s.c.) or 0.04 μg (i.m.) |
s.c. or i.m. |
Two doses (0 and 28 days) |
104 PFU VEEV TrD, 56 dpim |
aerosol or s.c. |
GMT PRNT80, ~60 to 2500 |
[160] |
|
VEEV |
F-iV3526 |
V3526 |
BALB/c mice, 8 to 10 weeks old |
1, 3 or 5 μg |
i.n., s.c. (under the skin over the neck) or i.m. (thigh muscle of the hind leg) |
Single dose |
454 (i.n.), 897 (i.m.) or 55 (s.c.) PFU VEEV-TrD, 56 dpim |
aerosol |
Microneutralization titer of 100 to 3500 |
|
|
Virus-like particle |
||||||||||
|
CHIKV |
VRC 311 Or VRC-CHKVLP059-00-VP/ PXVX0317 (in clinical trials) |
37997 |
BALB/c mice, 6 to 8 week old |
19 μg |
i.m. |
2 doses (2 and 5 weeks) |
ND |
ND |
IC50, 10703 to 54600 |
|
|
Cynomolgus macaques, 3 to 4 years old |
20 μg |
i.m. |
3 doses (0, 4 and 24 weeks) |
1010 PFU LR2006 OPY1, 15 wpim |
i.v. |
IC50, 10219 to 15072 |
||||
|
Human clinical trial, Phase 1 |
10, 20 or 40 μg |
i.m. |
3 doses (0, 4 and 24 weeks) |
NA |
NA |
IC50, 4525 to 8745 |
||||
|
Human, clinical trial Phase 2 |
20 μg |
i.m. |
2 doses (0 and 28 days) |
NA |
NA |
EC50 GMT, 2005 |
||||
|
Human clinical trial (Phase 2b, recruitment completed) |
6, 10 or 20 μg |
NP |
Two doses (0 and 14 or 28 days) |
NA |
NA |
NA |
||||
|
CHIKV |
Baculovirus-expressed VLP |
S27 |
AG129, 6 week old |
1 μg |
s.c. |
2 doses (0 and 21 days) |
1000 TCID50 S27, 6 wpim |
i.p. |
PRNT95, 40 to 80 |
|
|
C57BL/6 mice, 6 to 12 week old |
0.1 or 1 μg |
s.c. |
Single dose |
104 CCID50 LR2006 OPY1, 6 wpim |
s.c. |
NT95, ~1,100 |
||||
|
CHIKV |
Yeast-expressed VLP |
DRDE06/DRDE07 |
BALB/c mice, 4 week or 2 days old |
10, 20 or 40 ug |
s.c. |
Three doses (0, 14 and 28 days) |
ND |
ND |
NT50, 128 to 2048 |
[166] |
|
VEEV |
Venezuelan Equine Encephalitis Monovalent Virus-Like Particle Vaccine (VEEV) |
NA |
Human clinical trial (Phase 1, not recruiting) |
2, 10, or 20 μg |
i.m. |
Dose escalation (0, 28 days, and day 140 booster) |
NA |
NA |
NA |
[167] |
|
WEEV, EEEV, and VEEV |
VRC-WEVVLP073-00-VP (Trivalent vaccine) |
WEEV CBA87, EEEV PE-6 and VEEV TC-83 |
BALB/c mice, 6 to 8 week old |
monovalent (5 μg) or trivalent (5 μg each) |
i.m. |
Two doses (0 and 21 days) |
2.5 × 103 PFU WEEV CBA87, 8.9 × 103 PFU EEEV FL93-939, and 1.3 × 103 PFU VEEV Trinidad donkey, 56 dpim |
aerosol |
PRNT80, ~250 to 100000 |
[168] |
|
Cynomolgus macaques, age not specified |
Monovalent (20 μg) or trivalent (20 μg each) |
i.m. |
Two doses (0 and 28 days) |
106 PFU WEEV CBA87, 108 PFU EEEV FL93-939, and 108 VEEV Trinidad donkey, 56 dpim |
aerosol |
PRNT80, ~1000 to 10000 |
||||
|
Human clinical trial, Phase 1 |
6, 30 or 60 μg |
i.m. |
Dose escalation (0 and 8 weeks) |
NA |
NA |
NA |
[169] |
|||
|
DNA/RNA |
||||||||||
|
VEEV |
VEEV 26S DNA plasmid |
I/AB TrD |
BALB/c mice, 6 to 8 week old |
∼3 μg |
DNA/gene gun, delivered to two sites on the abdomen of each mouse |
Three doses (at 3-week intervals) |
∼104 PFU of TrD, 9 wpim |
s.c., aerosol |
PRNT50, GMT <1.6 to 2.5 |
|
|
Hartley guinea pigs, age not specified |
~5 μg |
DNA/gene gun, delivered to two sites on the abdomen of each mouse |
Three doses (0, 4 and 8 weeks) |
∼104 PFU of TrD, 21 wpim |
aerosol |
PRNT50, 0 to 640 |
||||
|
VEEV |
DNA-Ad |
TC-83 |
BALB/c mice, 6 to 8 week old |
1 μg of DNA per dose and 107 PFU of RAd/VEEV #3 per boost |
gene guni.n. |
immunised with the DNA vaccines on day 0, 14 and 28 and Ad-based vaccine on day 42 |
100 LD50 of virulent airborne VEEV, 63 dpim |
aerosol |
PRNT50, 160 |
[172] |
|
VEEV |
AG4-1C7 AG4-1G2 AG2-5A7 AG2-5A10 plasmid DNA |
I/AB TrD |
BALB/c mice, 6 to 8 week old |
4 μg |
particle-mediated epidermal delivery (i.d.) |
Three doses (at 3-week intervals) |
∼104 PFU of VEEV TrD (≥1000 LD50), 70 dpim |
aerosol |
PRNT80, ~1 to 5.5 log10 GMT |
[173] |
|
VEEV |
pTC83 iDNA |
TC-83 |
BALB/c mice, 3 week old |
50 μg |
i.m. electroporation |
Single dose |
105 PFU VEEV 3908, 21 dpim |
s.c. |
PRNT80, 10 to 320 |
[174] |
|
WEEV |
pE3-E2-6K-E1 pE3-E2 P6K-E1 |
71V-1658 |
BALB/c, age not specified |
2 μg |
gene gun |
Three doses (14 days apart) |
1500 PFU WEEV 71V-1658, Fleming, or CBA87, 42 dpim |
i.n. |
ND |
[175] |
|
CHIKV |
pCHIKV-Capsid, pCHIKV-Envelope (pMCE321) |
Consensus |
C57BL/6 mice, 3 to 4 week old |
25 µg, 2–3 times |
Electroporation |
Two doses (2 weeks apart) |
ND |
ND |
ND |
|
|
C57BL/6 mice, 6 to 8 week old |
25 μg |
i.m. electroporation |
Three doses (0, 14 and 21 days) |
7log10 PFU of PC-08, 35 dpim |
i.n. |
NP |
||||
|
BALB/c mice |
25 μg |
i.m. electroporation |
Two doses (2 weeks apart) |
7log10 PFU PC-08 |
i.n. |
TCID50, 20 to 320 |
||||
|
Rhesus macaques, age not specified |
1 mg |
i.m. electroporation |
Three doses (4 weeks apart) |
ND |
ND |
TCID50, 80 to 1280 |
||||
|
CHIKV |
Δ5nsP3 and Δ6K DNA |
LR2006 OPY1 |
C57BL/6 mice, 5 to 6 week old |
20 μg |
i.d. with DermaVax electroporation |
Single dose or two doses (0 and 3 weeks) |
106 PFU LR2006 OPY1, 7 wpim |
s.c. |
NT50, 100 to 10000 |
[131] |
|
CHIKV |
CHIKV-NoLS RNA |
LR2006 OPY1 |
C57BL/6 mice, 28 days of age |
2 μg |
s.c. in the ventral/lateral side of the right foot |
Single dose |
104 PFU LR2006 OPY1, 30 dpim |
s.c. in the ventral/lateral side of the right (ipsilateral) or left (contralateral) |
PRNT80, 0 |
[126] |
|
AG129 mice, 28 days old |
2 μg |
s.c. in the ventral/lateral side of the right foot |
Single dose |
104 PFU LR2006 OPY1, 30 dpim |
s.c. in the ventral/lateral side of the right (ipsilateral) or left (contralateral) |
ND |
||||
|
VEEV, WEEV and EEEV |
3-EEV |
VEEV IAB TrD, WEEV CBA874 and EEEV FL91-46794 |
C57BL/6 mice, 6 to 8 week old |
15 μg |
i.m. electroporation |
Two doses (0 and 21 days) |
104 PFU VEEV IAB TrD or 2 × 104 PFU WEEV CBA874 or 105 PFU EEEV FL91-46794, 7 wpim |
aerosol |
PRNT80, ~1 to 1000 |
[179] |
|
MAYV |
scMAYV-E |
NA |
C57BL/6 mice, 5 to 8 week old |
25 μg |
i.m. electroporation |
Single, two doses or three doses (at 2 week intervals) |
ND |
ND |
PRNT50, 789.8 |
[180] |
|
A129 mice, 4 to 6 week old |
25 μg |
i.m. electroporation |
Single, two doses or three doses (at 2 week intervals) |
102 PFU MAYV 15537 |
i.p. |
ND |
||||
|
CHIKV |
p181/25-7 |
TSI-GSD-28 |
BALB/c mice, 3 week old |
10 μg |
i.m. electroporation |
Single dose |
6 × 106 PFU CHIKV Ross, 28 dpim |
i.n. |
PRNT80, 160 to 1280 |
[181] |
|
CHIKV |
dMaB |
NA |
BALB/c mice, age not specified |
100 μg |
Electroporation |
Single dose |
107 PFU Del-03 |
s.c. or i.n. |
IC50, 3 to 4.5log10 |
[182] |
|
CHIKV |
iRNAΔ5nsP3 iDNAΔ5nsP3 |
LR2006 OPY1 |
C57BL/6 mice, 8 week old |
0.125, 1.25 or 10 μg |
i.m. in the gastrocnemius muscle of the left hind leg |
Single dose |
106 PFU LR2006 OPY1, 5 wpim |
s.c. at the dorsal side of each hind foot |
NT50, ~1 to 104 |
[183] |
|
VEEV |
pMG4020 DNA plasmid |
TC-83 |
BALB/c, 4 to 8 week old |
0.5 or 5 ug |
i.m. electroporation |
Single dose |
104 PFU VEEV TrD, 28 dpim |
s.c. |
PRNT80, 320 to >1280 |
[141] |
|
VEEV |
VEEVWT VEEVCOCAP VEEVCO |
IAB TrD |
BALB/c, 6 to 8 week old |
25, 5, or 1 μg |
i.m. electroporation |
Two doses (3 weeks apart) |
∼104 PFU VEEV IAB strain TrD, 7 wpim |
aerosol |
PRNT80, 1 to ~4.5log10 |
|
|
New Zealand White rabbits, age not specified |
500 μg of VEEVCO |
i.m. electroporation |
Three doses (0, 28 and 230 days) |
ND |
ND |
PRNT80, ~3log10 to 5log10 |
||||
|
Cynomolgus macaques, age not specified |
50 or 500 μg of VEEVCO |
i.m. electroporation |
Two doses (0 and 56 days) |
3 × 108 PFU VEEV IAB TrD |
aerosol |
PRNT80, ~0.8log10 to 3.5log10 |
||||
|
Human clinical trial, Phase 1 |
0.5 or 2 mg |
i.m. electroporation or i.d. electroporation |
Three doses (days 0, 28, and 56) |
NA |
NA |
GMT PRNT80, 7 to 78 |
||||
|
WEEV |
pVHX-671V-1658 pVHX-6 CBA87 pVHX-6 Fleming |
Fleming, CBA 87 or 71V-1658, |
BALB/c mice, age not specified |
2 shots × 2.5 μg precipitated on 0.5 mg gold |
gene gun |
Four doses (2 weeks apart) |
1.5 × 103 PFU WEEV Fleming, CBA 87 or 71V-1658, 8 wpim |
i.n. |
ND |
[186] |
|
WEEV and EEEV |
LANAC E1ecto |
WEEV McMillan |
CD-1 mice, 4 to 6 week old |
10 μg |
s.c. injection dorsal to the cervical spine |
Two doses (2 weeks apart) |
104 PFU WEEV McMillan, Montana-64, or EEEV Florida-93, 4, 5, 9, 11, or 13 wpim |
i.n. or s.c. |
PRNT50, <40 to 200 |
[187] |
|
CHIKV |
mRNA-1388 (or VAL-181388 in clinical trials) |
NA |
Human clinical trial, Phase 1 |
25, 50 or 100 μg |
i.m. |
Dose escalation procedure (0 and 4 weeks) |
ND |
ND |
‘dose-dependent increase’ in neutralizing and binding antibody titers |
[188] |
|
CHIKV |
mRNA-1944 |
SL15649 |
AG129, age not specified |
0.4, 1 or 10 mg/kg |
i.v. tail vein injection |
Single dose |
102.5 TCID50 of CHK |
subcutaneous injection in the footpad and hock of the right leg |
ND |
|
|
Cynomolgus macaques, 2 to 3 year old |
0.5 mg/kg |
i.v. |
Single dose |
ND |
ND |
FRNT50, 5 to 12 |
||||
|
Human clinical trial, Phase 1 (active, not recruiting) |
0.1, 0.3 and 0.6 mg/kg |
i.v. |
Dose escalation |
NA |
NA |
NT50, ‘all participants also showed circulating neutralizing antibody activity’ |
||||
|
Subunit |
||||||||||
|
CHIKV |
CHIKV-sE1 and -sE2 |
S27 |
AG129 mice, 6 week old |
2 μg |
s.c. |
Two doses (0 and 21 days) |
1000 TCID50 of S27 isolate, 9 wpim |
i.p. |
NT95, <25 |
|
|
CHIKV |
rE2p |
IND-06-AP3 |
BALB/c, 6 to 8 week old |
10, 20 or 50 μg |
i.m. |
Two doses (2 weeks apart) |
Mice immunized with 50 μg challenged with 7 log10 TCID50 /mL, 4 or 22 wpim |
i.n. |
NT50, 0.25log10 to 2.5log10 |
[154] |
|
CHIKV |
CHIKE1 and CHIKE2 recombinant proteins |
DRDE-06 |
BALB/c |
40 μg |
s.c. |
Three doses (0, 21 and 35 days) |
ND |
ND |
PRNT90, 32 to 512 |
[192] |
|
Chimeric virus |
||||||||||
|
Measles virus-based chimeras |
||||||||||
|
CHIKV (VLP) |
MV-CHIKV |
06–49 |
CD46-IFNAR, 6 week old |
103 to 105 TCID50 |
i.p. |
Single or two doses (30 days apart) |
100 PFU of CHIKV 06-49, 2 mpim |
i.p. |
PRNT50, 450 to 4050 PRNT90, 50 to 450 |
|
|
Cynomolgus macaques, age not specified |
5 × 105 (± 0.5 log) TCID50 |
i.m. |
Two doses (28 days apart) |
1.4 × 105 PFU LR2006 OPY1, 56 dpim |
s.c. |
PRNT80, 40 to >640 |
||||
|
Human clinical trial, Phase 1 |
1.5 × 104, 7.5 × 104 or 3.0 × 105 TCID50 |
i.m. or s.c. |
Dose escalation (0 and 28 days, or 0 and 90 days) |
NA |
NA |
PRNT50, 5 to 433 |
||||
|
Human clinical trial, Phase 2 |
5 × 104 or 5 × 105 TCID50 |
i.m. |
Three doses (0, 28, and 196 days) |
NA |
NA |
PRNT50, ~5 to 5000 |
||||
|
Alphavirus-based chimeras |
||||||||||
|
CHIKV |
VEE/CHIKV EEE/CHIKV SIN/CHIKV |
LR2006 OPY1 |
NIH Swiss, C57BL/6, >3 week old |
5.8 log10 PFU (VEE/CHIKV and SIN/CHIKV), 5.3 log10 PFU (EEE/CHIKV) |
s.c. in the medial thigh |
Single dose |
6.5 log10 PFU (Ross CHIKV strain), 21 dpim |
i.n. |
PRNT80, 20 to 320 |
[198] |
|
CHIKV |
VEE/IRES-CHIKV VEE/IRES-C/CHIKV |
NA |
A129 mice, 6 to 9 week old |
104 PFU |
s.c. |
Single dose |
102 PFU of LR2006 OPY1, 5 weeks post immunization |
s.c. |
PRNT80, >640 |
[199] |
|
CHIKV |
EILV-CHIKV |
CHIKV 996659 |
C57BL/6 mice, 4 week old |
8.8 log10 PFU |
s.c. |
Single dose |
6 log10 PFU 99659, 30 dpim |
i.d. |
PRNT80, ≥ 80 |
|
|
IFNα/βR−/−, 6 week old |
8.8 log10 PFU |
s.c. |
Single dose |
3 log10 PFU 99659, 292 dpim |
i.d. |
PRNT80, 160 to 1280 |
||||
|
Cynomolgus macaques, 3 to 5 years |
8.1 log10 PFU |
i.m. into the right quadriceps |
Single dose |
5 log10 PFU LR2006 OPY1, 31 dpim |
s.c. |
PRNT80, 80 to 640 |
||||
|
EEEV |
EILV/EEEV |
EEEV FL-93 |
Adult CD-1 mice (age not specified) |
108 PFU |
s.c. |
Single dose |
105 PFU EEEV-FL93, 70 dpim |
i.p. |
PRNT80, 80 to 640 |
|
|
EEEV |
Trivalent EILV/EEEV EILV/VEEV EILV/CHIKV |
EEEV FL-93, VEEV IAB TC-83, CHIKV 996659 |
Adult CD-1 mice (age not specified) |
108 PFU |
s.c. |
Single dose |
105 PFU EEEV-FL93, 70 dpim |
i.p. |
PRNT80, 40 to 640 and 20 to 640 for mono- and trivalent vaccines respectively |
|
|
VEEV |
EILV/EEEV |
VEEV IAB TC-83 |
Adult CD-1 mice (age not specified) |
108 PFU |
s.c. |
Single dose |
103 PFU VEEV-IC 3908, 70 dpim |
s.c. |
PRNT80, 80 to 1280 |
|
|
VEEV |
Trivalent EILV/EEEV, EILV/VEEV EILV/CHIKV |
EEEV FL-93, VEEV IAB TC-83, CHIKV 996659 |
Adult CD-1 mice (age not specified) |
108 PFU |
s.c. |
Single dose |
103 PFU VEEV-IC 3908, 70 dpim |
s.c. |
PRNT80, 40 to 640 and 20 to 80 for mono- and trivalent vaccines respectively |
|
|
EEEV (Sindbis virus) |
SIN/NAEEEV |
EEEV FL93-939 |
NIH Swiss mice, 8 week old |
3.7, 4.7 or 5.7 log10 PFU |
s.c. |
Single dose |
6 log10 PFU FL93-939, 28 dpim |
i.p. |
PRNT80, 125 to 660 |
[200] |
|
SIN/SAEEEV |
EEEV BeAr436087 |
NIH Swiss mice, 8 week old |
3.8, 4.8 or 5.8 log10 PFU |
s.c. |
Single dose |
6 log10 PFU FL93-939, 28 dpim |
i.p. |
PRNT80, 28 to 308 |
||
|
VEEV |
SIN-83 |
VEEV IAB TC-83 |
Weanling NIH Swiss mice, 6 day old |
103, 104, 105 or 106 PFU |
s.c. |
Single dose |
106 PFU VEEV IC ZPC738 IC SH3 |
s.c.in medial thigh |
PRNT80, 30 to 960 |
|
|
NIH Swiss mice, 6 week old |
5 × 105 PFU |
s.c. |
Two doses |
2 x 105 or 106 PFU VEEV ZPC738, 8 wpim |
s.c., i.c., or i.n. |
PRNT80, 55 to 73 (single), 100 to 160 (booster) |
||||
|
SAAR/TRD |
VEEV IAB TrD |
NIH Swiss mice, 6 week old |
5 × 105 PFU |
s.c. |
Two doses |
2 x 105 or 106 PFU VEEV ZPC738, 8 wpim |
s.c., i.c., or i.n. |
PRNT80, 126 to 167 (single), 152 to 160 (booster) |
||
|
SIN/TRD |
VEEV IAB TrD |
NIH Swiss mice, 6 week old |
5 × 105 PFU |
s.c. |
Two doses |
2 x 105 or 106 PFU VEEV ZPC738, 8 wpim |
s.c., i.c., or i.n. |
PRNT80, 37 to 57 (single), 50 to 73 (booster) |
||
|
SIN/ZPC |
VEEV ID ZPC738 |
NIH Swiss mice, 6 week old |
5 × 105 PFU |
s.c. |
Two doses |
2 x 105 or 106 PFU VEEV ZPC738, 8 wpim |
s.c., i.c., or i.n. |
PRNT80, 187 to 253 (single), 253 to 487 (booster) |
||
|
All the above |
VEEV IAB TC-83, IAB TrD, ID ZPC738 |
Syrian golden hamsters, 6 week old |
5 × 105 PFU |
s.c. in the medial thigh |
Single dose |
106 PFU |
s.c.in medial thigh |
ND |
||
|
WEEV |
SIN/CO92 |
WEEV CO92-1356 |
NIH Swiss mice, 6 week old |
3.5, 4.5, or 5.0 log10 PFU |
s.c. in the medial thigh |
Single dose |
5.3 log10 PFU WEEV TBT235, 28 dpim |
i.n. |
PRNT80, 20 to 640 |
[203] |
|
SIN/SIN/McM |
WEEV McMillan |
NIH Swiss mice, 6 week old |
4.8 or 5.8 log10 PFU |
s.c. in the medial thigh |
Single dose |
5.0 log10 PFU WEEV McMillan, 28 dpim |
i.n. |
PRNT80, 600 to 604 |
||
|
SIN/EEE/McM |
EEEV 436087 and WEEV McMillan |
NIH Swiss mice, 6 week old |
4.6 or 5.6 log10 PFU |
s.c. in the medial thigh |
Single dose |
5.0 log10 PFU WEEV McMillan, 28 dpim |
i.n. |
PRNT80, 416 to 420 |
||
|
Vaccinia virus-based chimeras |
||||||||||
|
CHIKV |
MVA-CHIKV |
LR2006-OPY1 |
C57BL/6 mice, 6 to 8 week old |
107 PFU (first dose), 2 × 107 PFU (second dose) |
i.p. |
Two doses (2 weeks apart) |
106 PFU LR2006-OPY1, 9 wpim |
s.c. in the dorsal side of each hind foot |
NT50, ~100 to 3000 |
[204] |
|
CHIKV |
MVA-CHIK |
LR2006-OPY1 |
BALB/c mice, 4 to 6 week old |
107 TCID50 units |
i.d. injection into the left hind footpad. |
Single or two doses (28 days apart) |
104 LR2006 OPY1 TCID50 units at 39 or 42 dpim |
i.d. |
TCID50, 5 to 15 |
[205] |
|
AG129, 6 to 10 week old |
107 TCID50 units |
i.d. injection into the left hind footpad. |
Single or two doses (28 days apart) |
102 LR2006 OPY1 TCID50 units at 39 or 42 dpim |
i.d. |
TCID50, 4 to 8 |
||||
|
CHIKV |
MVA-6KE1, MVA-E3E2, MVA-6KE1E3E2 |
CHIKV S27 |
AG129 mice, 7 week old |
5 × 106 TCID50 |
i.m. into the quadriceps muscles of the left leg |
Two doses (3 weeks apart) |
103 TCID50 CHIKV-S27 and CHIKV-IND/NL10, 63 dpim |
i.p. |
NT100, 10 to 160 |
[206] |
|
EEEV, VEEV, and WEEV |
MVA-BN-E/V/W (monovalent) MVA-BN-E + MVA-BN-V + MVA-BN-W (triple mixture of monovalent vaccines) MVA-BN-WEV (trivalent) |
WEEV 71 V-1658, EEEV FL93-939NA and VEEV TrD |
BALB/c mice, age not specified |
108 TCID50 |
s.c. or i.m. |
Two doses (28 days apart) |
5 × 103 or 104 PFU of WEEV Fleming, EEEV PE6, or VEEV TrD, 14 days post booster |
i.n. |
NT50, ~750 to 3800 (monovalent), ~<60 to 340 (triple mixture of monovalent vaccines) and ~<60 to 380 (trivalent) |
[207] |
|
Adenovirus-based chimeras |
||||||||||
|
CHIKV |
CAdVax-CHIK |
LR2006 OPY1 |
CD-1 or C57BL/6, 6 to 8 week old |
108 IU |
i.p. |
Single dose |
104 CCID50 LR2006 OPY1 or QIMR, 6.5 wpim |
s.c. into side of each hind foot towards the ankle |
NT100, ~2000 |
[208] |
|
CHIKV |
ChAdOx1 Chik |
NA |
BALB/c, 6 to 8 week old |
108 IU |
i.m. |
Single dose |
ND |
ND |
NT50, 5.39 × 103 |
|
|
AG129, 5 week old |
108 IU |
i.m. in each leg |
Single dose |
9.7 × 104 PFU LR2006 OPY1, 30 dpim |
i.d. into the left foot |
ND |
||||
|
ChAdOx1 Chik ChAdOx1 Chik ΔCap |
AG129, 5 week old |
108 IU |
i.m. in each hind leg |
Single dose |
9.7 × 104 PFU of LR2006 OPY1, 30 dpim |
i.d. into the left foot towards the ankle |
PRNT80, 32 to 64 (Chik), 16 to 32 (Chik ΔCap) |
[211] |
||
|
CHIK001 (in clinical trials) |
Human clinical trial, Phase 1 |
5 × 109, 2.5 × 1010 or 5 × 1010 vp |
i.m. |
Single dose |
ND |
ND |
ND |
[212] |
||
|
MAYV |
ChAdOx1 May |
NA |
AG129, 5 week old |
1.6 × 104 PFU |
i.m. in each leg |
Single dose |
1.6 × 104 PFU MAYV-CH, 30 dpim |
i.d. into the left foot |
PRNT50, 160 to 620 |
[210] |
|
VEEV |
Rad/VEEV#3 |
VEEV IAB TC-83 |
BALB/c, 6 to 8 week old |
107 PFU |
i.n. |
Three doses (at 0, 7 and 21 days) |
Dose ND, 28 dpim |
aerosol |
PRNT50 (NP) |
[213] |
|
BALB/c, 6 to 8 week old |
107 PFU |
i.n. |
Two doses (at 0, 21 days) |
5000 LD50 TrD, 42 dpim |
aerosol |
ND |
[214] |
|||
|
WEEV |
Ad5-WEEV |
WEEV 71V-1658 |
BALB/c mice, age not specified |
107 PFU |
i.m. |
Single or two doses (at 4 weeks) |
1.5 × 103 PFU Fleming or 71V-1658, 13 wpim |
i.n. |
PRNT50, 160 |
[215] |
|
WEEV |
Ad5-E1 |
WEEV 71V-1658 |
BALB/c mice, 6 to 9 week old |
107 PFU |
i.m. in both leg |
Single dose |
50 LD50 of 71V-1658, 7 dpim, or 400 LD50 CBA87, 1, 3, 5 or 7 dpim |
i.n. |
PRNT50, <10 |
[216] |
|
Vesiculovirus-based chimeras |
||||||||||
|
CHIKV |
rVSVΔG-CHIKV |
CHIKV S27 |
C57BL/6, 3 week old |
106 PFU |
i.m. into the right hind leg muscle |
Single dose |
104 PFU LR 2006 OPY1, 30 dpim |
s.c. in the left rear footpad |
PRNT80, 80 to 640 |
[217] |
|
VEEV |
rVSIV-VEEV |
VEEV ZPC738 |
CD-1, 4 to 6 week old |
108/107 PFU |
i.m. |
Single dose |
104 PFU VEEV ZPC738, 35 or 245 dpim |
s.c. |
PRNT80, 288 to 600 at 25 and 35 dpim, 304 to 360 at 245 dpim |
[218] |
|
VEEV |
rISFV-VEEV |
VEEV ZPC738 |
CD-1, 4 to 6 week old |
108 PFU |
i.m. |
Single dose |
104 PFU VEEV ZPC738, 28 dpim |
s.c. |
PRNT80, ≥20 |
|
|
CD-1, 4 to 6 week old |
108 PFU |
i.m. |
Single dose |
104 PFU VEEV ZPC738, 35 or 245 dpim |
s.c. |
PRNT80, 40 to 160 at 25 and 35 dpim, 25 to 64 at 245 dpim |
||||
|
EEEV |
rISFV-EEEV |
EEEV FL93-939 |
CD-1, 4 to 6 week old |
108 PFU |
i.m. |
Single dose |
104 PFU EEEV FL93-939, 28 dpim |
s.c. |
PRNT80, ≥20 |
|
|
Epitope-based |
||||||||||
|
CHIKV |
E2EP3 |
NA |
C57BL/6 mice, 3 week old |
100 μg (50 μg for booster doses) |
s.c. in the abdominal flank |
Three doses (0, 14 and 21 days) |
106 PFU CHIKV SGP11, 30 dpim |
s.c. region at the ventral side of the right hind footpad, towards the ankle |
~40% reduction from mock control |
[23] |
1 s.c., subcutaneous; i.v., intravenous; i.m., intramuscular; i.d., intradermal; i.p., intraperitoneal; i.n., intranasal; i.t./i.s., intrathalamic/ intraspinal; i.pl., intraplantar; i.c., intracranial; dpim, days post immunization; wpim, weeks post immunization; mpim, months post immunization; IRES, internal ribosome entry site; PFU, plaque forming units; TCID50, 50% tissue culture infective dose; CCID50, 50% cell culture infectious dose; IC50, 50% inhibitory concentration; GE, genomic equivalents; IU, infectious units; AID50, 50% animal infectious dose; PRNT50, 50% plaque reduction neutralizing antibody titer; PRNT80, 80% plaque reduction neutralizing antibody titer; PRNT90, 90% plaque reduction neutralizing antibody titer; LD50, median lethal dose; NT50, 50% neutralizing titer; GMT, geometric mean titer; μNT, neutralizing titer; SIN, Sindbis virus; ISFV, Isfahan virus; May, Mayaro virus; EILV, Eilat virus, VSV/VSIV, vesicular stomatitis virus; MV, measles virus; MVA, modified vaccinia virus Ankara; NP, not provided; NA, not applicable; WT, wild type. Data curated from literature reported through February 2021.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9050899
