1. Background
Searching for sustainable, clean, and highly efficient energy is the main method for solving the energy crisis and environmental pollution problems brought about by the first and second industrial revolutions, which have built a modern and prosperous society based on carbon-based fuels [
1,
2,
3,
4]. Human actions have led to the carbon dioxide content in the atmosphere rising rapidly and exceeding 400 ppm currently, mostly originating from the burning of coal, oil, and gas [
2,
5]. The application of wind and solar power and other types of renewable electricity generation technologies seems to be an efficient way to fulfill the requirement of an energy revolution [
6,
7,
8]. However, they are strongly intermittent in nature due to diurnal or seasonal variations [
9]. Converting their energy to a zero-emission chemical energy carrier such as hydrogen is an alternative that can achieve versatile utilization, such as clean heating or electricity at a later stage, on account of the high energy density of hydrogen [
5,
10,
11,
12]. Therefore, an increasing number of sustainable pathways for energy conversion and storage technologies, including water electrolysis, batteries, and fuel cells, have been proposed and extensively investigated [
5,
13,
14,
15]. Proton exchange membrane water electrolysis (PEMWE) operating in acidic environments has offered an effective way to produce sustainable, high-purity hydrogen through targeted electrochemical reactions since the 1960s [
16] (). PEMWE has the advantages of a faster dynamic response, a higher current density, and lower crossover of gases and is considered to be the basis of a hydrogen society in the future [
17,
18,
19].
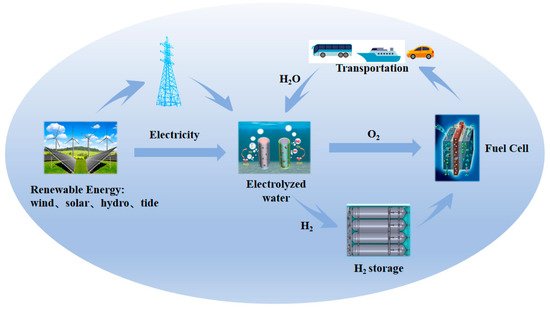
Figure 1. Schematic of sustainable pathways for energy conversion and storage based on electrocatalysis.
Electrochemical water splitting involves two heterogeneous multi-step half-reactions, which are referred to as the cathodic hydrogen evolution reaction (HER) and the anodic oxygen evolution reaction (OER) [
20,
21]. Owing to the inherent energy barrier, the practical operating voltage of commercial water electrolyzers is higher than the theoretical 1.23 V (versus a reversible hydrogen electrode) under the standard conditions (298 K and 1 atm) [
22,
23]. For example, industrial electrolytic water generally maintains the external voltage at 1.8~2.0 V [
16]. Typically, the descriptor of overpotential is used to show the difference between the thermodynamic potential and the practical potential required to drive the electrochemical reaction [
24]. The overpotential mainly comes from the electrochemical polarization on the anode side (
ηa) and cathode side (
ηc) and the ohmic polarization caused by other resistors (
ηother) [
25]. Comparing
ηa and
ηother, the intrinsically sluggish kinetics of the OER involving a four electron–proton coupled reaction (Equation (1)) hampers the overall water-splitting process [
16,
26].
One solution to this conundrum is to develop suitable catalysts with high efficiency and low overpotential [
27]. However, most of the excellent OER catalysts with high activity and durability are not stable in acidic solutions [
28]. They are easily oxidized and decomposed in a strong acid system, which is one of the indispensable working conditions for PEMWE [
29]. Currently, the iridium (Ir) and ruthenium (Ru)-based electrocatalysts are regarded as the state-of-the-art commercial electrocatalysts for the OER [
30,
31]. Compared with other catalysts, they exhibit excellent OER catalytic activity due to their inherent promising activity, even if severe corrosion still exists under strong acid working conditions [
32]. This provides a driving force for the vast majority of studies on the modifications of these electrocatalysts, including composition, structure, and morphology optimizations [
23,
33,
34,
35]. Outstanding OER electrocatalysts should have excellent intrinsic activity and sufficient active sites [
18], and these requirements are generally combined with simplicity and controllability. In this regard, optimizations of the reaction energy barrier, electronic conductivity, and reaction surface area of the OER electrocatalysts are of great importance [
18,
36,
37]. The transport efficiencies of electrons, ions, and produced oxygen are directly related to the number of channels, which depend on rational surface/interface engineering through nanostructural modifications, such as pore size control and construction of a multi-stage structure [
33,
38]. The nanostructures include zero-dimensional nanoclusters, nanoparticles, nanocages, and nanoframes [
39]; one-dimensional nanotubes and nanowires [
40]; two-dimensional nanosheets [
41]; and three-dimensional nanowire networks, aerogels, etc. [
42,
43]. Moreover, simple and effective surface/interface engineering techniques have been diversified for adjusting the surface atoms, electronic structures, interfacial stresses, and bridge bonds, such as doping elements, tailoring the coordination environment, and loading with active materials [
43,
44,
45,
46].
Although Ru/Ir-based electrocatalysts have indeed shown good OER performance, they are still far from ideal OER electrocatalysts in terms of activity and are not completely stable at high oxidative potentials [
17,
47]. A growing body of evidence shows that Ru-based OER catalysts dissolve extensively during the electrocatalytic process [
32]. This is because the onset potential of Ru-based catalysts is consistent with the corrosion potential of the metal Ru [
48]. Ir-based catalysts also suffer similar degradation issues [
49]. During the long-term catalytic process, rutile oxide of IrO
x will transition to other kinds of phases that are soluble in acid media [
50]. Therefore, the harsh operating conditions must be taken into account when designing suitable catalysts. Based on this, substantial research efforts have been devoted to investigating the low-precious-metal or precious-metal-free OER catalysts that are stable in acid media, such as perovskite, spinel, and the layer-structure-type family [
23,
51,
52]. These kinds of catalysts also exhibit remarkable activity and are low-cost, easily synthesized, and environmentally benign [
23].
2. Mechanisms for the OER in Acidic Media
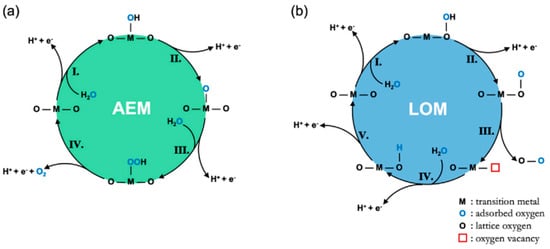
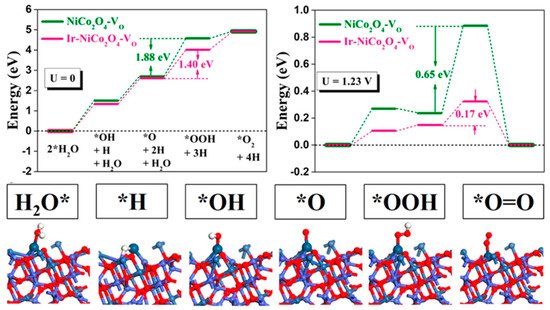
In the case of the OER in acid media, two possible mechanisms built on consecutive proton and electron transfers during the catalytic cycle, known as the adsorbate evolution mechanism (AEM) and the lattice oxygen participation mechanism (LOM), have been widely accepted [
24,
26,
54,
55] (). For the reaction path based on the AEM, a water molecule first adsorbs on a surface metal cation and decomposes into a proton (H
+) to form HO*, which further dissociates the second proton to form O* in the second step. After that, HOO* is formed by the nucleophilic attack from another water molecule on the O*. Finally, oxygen is released, accompanied by the desorbed proton. Another four-electron transfer mechanism, known as the LOM, has been proposed based on a series of in-situ isotopic labeling experiments. In contrast to the AEM, lattice O participates in the formation of oxygen for the LOM. Firstly, one water molecule is adsorbed on a surface lattice O and dissociates the first proton to form HO*, which further dissociates the second proton to form O* in the second step. After that, oxygen is released via coupling absorbed O and a surface lattice O along with the presence of a surface oxygen vacancy. Finally, the surface lattice is restored as before via water adsorption and dissociation on the vacancy [
24].
Figure 2. Diagrammatic sketch of (
a) the AEM mechanism proposed by Rossmeisl et al. and (
b) the LOER mechanism proposed by Rong et al. [
24].
Although both of the mechanisms involve a four-electron transfer, there are still some differences between them [
26]. The first one is that the AEM requires a higher reaction energy barrier (>320 mV) than the LOM theoretically [
56]. In terms of active sites, the catalytic process predominantly involves a cationic redox (i.e., transition metal ions) in the AEM mechanism and an anion redox (i.e., lattice oxygen) in the LOM [
57]. However, it varies according to actual conditions. Jones et al. demonstrated that both mechanisms exist in the OER, which can be detected by the charge storage behavior via the applied bias [
58]. At a low bias, it mainly involves the charge storage of metal centers; at a high bias, it involves the storage of oxygen in IrO
x. Moreover, strategies for increasing activity are different based on these two different mechanisms. On basis of the AEM, the active metal centers are always at a lower valence state, which can promote the nucleophilic attack of water molecules by increasing the covalence of metal and oxygen [
43]. For example, Stoerzinger et al. simulated under-coordinated Ru atoms on a well-crystallized RuO
2 surface with superior OER activity [
59]. For the LOM, the metal center is often at a higher valence state, which is committed to promoting the generation of more electrophilic oxygen atoms and increasing the interaction between metal and oxygen [
60]. Tarascon et al. studied the lattice oxygen behavior of La
2LiIrO
6. They believed that Ir was not the active site for the OER owing to the pH-dependent activity [
61]. Despite the existing difference, some phenomena occurring in the process of an OER can still be explained by these theories. For example, excessive oxidation of metal sites for the AEM and lattice participation for the LOM generally lead to material instability [
49]. Furthermore, the reason why the amorphous metal oxides exhibit better catalytic activity is that lattice oxygen can participate in the catalytic reaction easier than the well-crystallized ones [
35].
It should be mentioned that, apart from increasing the site density, we can also optimize the composition to modify the intrinsic activity of the OER [
33,
62]. Recent work has pointed out the superiority of bi-metal oxides as some of the most advanced electrocatalysts toward the OER in acidic media, in terms of features including decreased Ru/Ir contents and enhanced OER activity and selectivity [
23,
63]. Incorporating suitable foreign metal atoms, such as Cr, Ni, Zn, and Cu atoms, can surprisingly improve the conductivity and alter the electronic structures of the original catalysts, thus enhancing their intrinsic activity [
64,
65]. It was reported that a low Ru content oxide material (Cr
0.6Ru
0.4O
2) derived from a Cr-based metal–organic framework showed remarkable OER performance in acidic media [
66]. The superior catalytic activity and stability can be assigned to the lower occupation at the Fermi level and the altered electronic structures by incorporating Cr. Regarding Ni-modified oxides, it is suggested that Ni serves as the sacrificial component, as its leaching generally leads to enhanced OER activity due to the formation of active OH-containing surface structures [
67]. For instance, Ni leaching was observed during the OER process of bulk Ir–Ni mixed oxides with increased Ni contents (21 atomic%, 39 atomic%, and 89 atomic%), and the remaining Ni concentrations were similar in all systems, which turned out to be ~12 atomic% relative to the total amount of Ir and Ni in the oxides [
68]. However, differences in their OER performance suggested variations in the resulting active sites caused by the sacrifice of Ni. This means that the OER performance of the catalysts is directly related to the transport kinetics of the electrons involved, and the reaction rate is determined also by the number of active sites. Therefore, it is important to prepare electrocatalysts with a sufficient reaction surface area in order to enable facile mass/electron transport and alter the interaction between metals and supports [
69]. The most effective way is to minimize the size of catalyst nanoparticles to within several nanometers to make full use of each active site [
36]. In addition, composition modification may also increase the number of catalytic sites. Doping Zn and Cu can confer moderate binding strength on oxygen intermediates, provide more defects or vacancies to enhance the intrinsic activity, and significantly increase the surface area to expose more active reaction sites [
70]. However, difficult issues such as well-controlled monodispersity, stabilization of active sites, targeted synthesis, and macro-scale configuration for OER electrocatalysts still remain, especially in acidic media, both experimentally and theoretically [
23,
71].