Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Carbon in its various forms (e.g., nanotubes, fullerenes, graphene) create a family of substances that enable the storage of large amounts of hydrogen in a reversible manner, which is confirmed by both computer simulations and experimental results.The main contraindication to using hydrogen as an energy source in mobile applications is still the low gravimetric density achieved by the available systems (according to the recommendations of the US Department of Energy, it should be 6 wt.%).
- hydrogen storage
- carbon-based materials
- green energy
- graphene
1. Hydrogen in energy industry
In our daily life, energy is one of the most integral and necessary elements for us to function. As a result, the constantly growing consumption of energy from various sources is an easy trigger for the emergence of an energy crisis in the area of the whole world. This crisis is related to both the oversupply and demand for energy in every area of life, as well as the constant use of fossil fuels for this purpose. This is mainly due to the constantly growing world population, as well as the growing demand for new technologies and rising living standards in the world. Also, most of the world’s economies are based primarily on the use of non-renewable energy sources [1],[2].
It is estimated that up to 80% of the total energy used in the world is the energy obtained from mainly three sources: coal, natural gases, and petroleum. These sources are not inexhaustible, however, the energy crisis related to the use of these fuels may have its climax already in 2035 and last for several years. Limited sources of fossil fuels and rising oil prices, combined with the growing greenhouse gas emissions, pose a challenge to the search for new energy sources and the possibility of using it in many branches of the energy industry [3]. The impact of the automotive industry is particularly important in terms of the excessive use of fuels and the related emission of harmful greenhouse gases and other products of combustion of these fuels into the atmosphere. According to estimations prepared for year 2050, the demand for oil or gas can triple in relation to the current needs [4].
Hence, by observing the trends in the growing needs of the energy industry, scientists are looking for alternatives to traditional energy sources with the benefit of sustainable methods as an alternative fuel source. On this basis, several years ago, work began to intensify on the use of hydrogen as a sustainable energy source [5],[6],[7]. On Figure 1, there are presented factors that might promote or inhibit the use of hydrogen as an energy source in not too distant future.
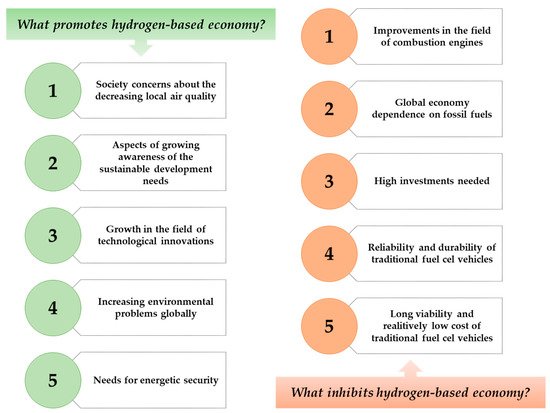
Figure 1. Factors that inhibit and promote the development of hydrogen-based economy.
Hydrogen is an element of a great importance. As it is the simplest, the lightest, and the most abundant particle on earth, its accessibility makes it a perfect possible solution in terms of many industrial applications, including its use in energy industries. The use of this element has many advantages with very low number of disadvantages, and all these are mentioned in Figure 2.
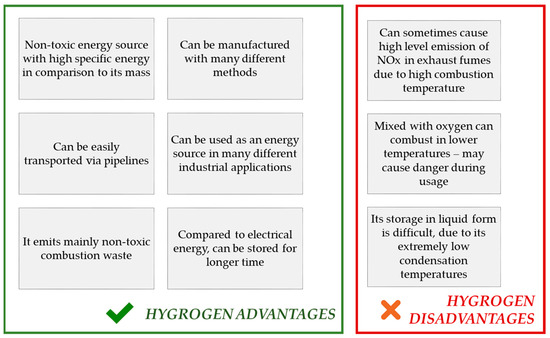
Figure 2. Advantages and disadvantages of H2 as an energy source.
However, even if hydrogen can be produced via diverse methods, still the requirements on the possibilities of the most effective hydrogen storage has to be exploited.
2. Hydrogen Storage—Challenges, Methods, Applications
One of the most difficult challenges for using hydrogen as an energy source is its storage. When designing possible solutions in this area, first of all, one should take into account both economic and purely technological aspects [8].
It can be easily noticed that due to the physical characteristics of hydrogen atoms, the need to find a material for its most efficient storage is a challenging task. The material’s solution for this purpose must fulfill several technical aspects to be considered for further industrial applications. Hydrogen can be stored in three ways[9].
-
Chemically, with the help of different liquid or solid chemical compound;
-
Physically (due to the changes of its physical state conditions like pressure, temperature, or phase;
-
Or physico-chemically, combining the previous methods.
The division of ways on how to store hydrogen is presented in Figure 3.
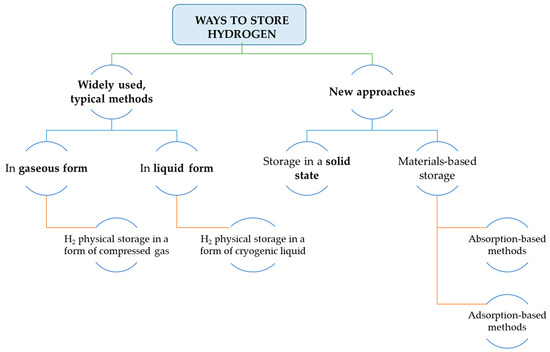
Figure 3. Approaches to store hydrogen (on the basis of [10]).
A great challenge in hydrogen storing is to allow it to be collected in liquid or solid form. Traditional methods of storing hydrogen, both for stationary and mobile applications, have some limitations, especially due to the very low boiling point (20.2 K) and the extremely low density of hydrogen (0.09 kg/NA m³) in the gaseous and exceptionally high density (70.9 kg/NA m³) in the liquid phase. Additionally, the methods currently used are limited by other factors. First of all, the disadvantage of the methods used is the significant energy loss, which amounts to 40% in the case of condensation and up to 20% of the hydrogen content needed to compress the gas. Moreover, a very important issue is also the necessity to limit the use of high-pressure and cryogenic storage, mainly due to the social aspects that negatively associate the use of gas under pressure and difficulties with stopping the release of liquid H2 [11].
In recent years, several phenomena and methods has been applied to fulfill the needs of stationary hydrogen storage. Among them, the most popular ones are listed in Figure 4.

Figure 4. Phenomena and methods used in hydrogen storage methods developed over the past several years (on the basis of [12]).
A significant technological breakthrough is needed in order to make hydrogen storage efficient and the most economically effective alternative compared to compressed and liquid hydrogen. This means either its solid or liquid storage. However, there is an urgent need to improve the H2 absorption and desorption properties in storage materials, mainly due to the fact that its storage in liquid form is difficult because hydrogen condensation requires very low temperatures.
Taking this into consideration, the most promising routes for hydrogen storage are solid materials, whose role will be to chemically bind or physically adsorb hydrogen at bulk densities greater than liquid hydrogen. The scheme in Figure 5 presents the most popular methods for hydrogen storage and the mechanisms behind it.
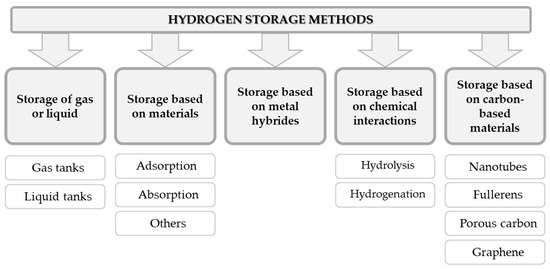
Figure 5. Summary of the most popular hydrogen storage methods [13].
Industrial Applications for Hydrogen Storage
The literature review of the current state-of-the-art in terms of hydrogen use and the hydrogen storage technologies, points out that in not distant future, the use of H2 as an energy source will be present in almost all areas of our everyday life [14].
For now, industrial use of this gas is mostly focused on ammonia production, petroleum, and metals refining [15]. Over these applications, the growing interest in replacing fossil fuels by hydrogen has been intended, mostly in the automotive area.
The need to find an optimized way for hydrogen storage, especially for mobile applications, has originated mostly from the issue of really low density of this gas which results in the need for inadequately large surface area of the fuel tank [16]. In the area of the use of hydrogen as an energy source e.g., in automotive industry, there has been distinguished two ways of performance, one including the rapid combustion of hydrogen in an engine, together with oxygen from the atmosphere, and one in which electrochemically burnt H2 and O2 (originated from fuel cell) are releasing electrical and heat energy. Both these methods allow the engine to start working, but the problematic issue is not the way how this is supposed to work, but the demanded size for the hydrogen storage area.
Taking these into consideration, it can be clearly seen that storing hydrogen for the mobile purposes still poses a major research challenge for materials engineers. The main difficulties come from the need to achieve a storage medium that enable to store hydrogen with its great density, combined with fast, reversible kinetics of charging and discharging. To achieve this, the considered material requires strong chemical bonds and close atomic packaging. However, to provide a demanded kinetics of adsorption and desorption, requirements are focused on weakly bonded material structures, brittle at moderate temperatures. Moreover, to facilitate a rapid diffusion of hydrogen, loose atomic packaging of storage material and adequate thermal conductivity (in order to prevent heat induced degradation) are needed. By now, scientists seek a way to fulfill all these requirements. From several years, the novel ways developed to serve these purposes come from the field of nanosciences. The possible use of nanosized materials, especially with large surface area hybrid structure, allows the demanded multifunctional action of material serving as the hydrogen storage source [17].
3. General Characteristic of Carbon-Based Materials Concerning Hydrogen Storage
Graphene belongs to two-dimensional materials, sometimes referred to as single layer materials. It is a class of nanomaterials merely one atom thick. These materials are extracted from layered materials with strong in-plane chemical bonds and weak coupling between the individual layers or artificially synthesized on the substrate. Experimentally, it was first developed via scotch tape peeling of graphite by Andre Geim and Konstantin Novoselov [18]. In its pure form, graphene is a sheet of carbon atoms arranged in hexagon network resulting from sp2 hybridization. Material in such form has an obvious tendency to restack and agglomerate. In general, graphite can be treated as numerous layers of graphene that are arranged parallelly via van der Waals bonds that maintain the distance between the planes equal to 0.335 nm [19]. The layers can also be bent into fullerenes or nanotubes. In the first case, the closed ball-like mesh of rings of five to seven atoms originates from carbon atoms that are connected not only via single but also double bonds. In case of nanotubes, the structure is cylindrical like and can be either closed or opened at the ends. That common name refers to single-wall carbon nanotubes (SWCNTs) as well as multi-wall carbon nanotubes (MWCNTs) consisting of nested single-wall carbon nanotubes (CNTs).
Graphene, its properties, and available applications are highly dependent on the manufacturing method used to obtain it. Exfoliation is a process of separation of the layers of the material that can be conducted micromechanically (like during graphene discovery) or in liquid phase. Large-scale production of graphene using this method is rather problematic, which stays against its common use for hydrogen storage. In case of use of chemical vapor deposition (CVD), the manufacturing requires formation of graphene on the substrate due to segregation of carbon atoms dissolved in metals with high solubility of that element [20] or direct nucleation and further expansion on materials with low carbon solubility [21]. Graphene obtained in that manner is of high quality, but similarly to the one obtained by epitaxial growth on SiC [22] or pulsed laser deposition [23], its surface is small. In case of high strength metallurgical graphene (HSMG), the key point of achieving both high quality and large area is the growth of graphene on a liquid metallic matrix [24]. Nevertheless, the obtained material is in the form of sheets that require additional procedures of spatializing [25] to make it applicable for hydrogen storage in, for example, automotive industry. Graphene at mass scale is produced by Hummers’ method based on a multi-chemical treatment of graphite to obtain graphene oxide powders [26]. Probably such form of graphene, after necessary modification, is the most promising for hydrogen storage concerning the low costs of mass production.
The hydrogen storage in carbon-based materials is based both on the physisorption and chemisorption. Physisorption usually occurs with hydrogen in the molecular form and behind its mechanism is the weak interactions with the surface like those based on van der Waals forces, electrostatic and dispersion interactions [27]. The theoretical physisorption of hydrogen on single plane of graphene forming graphite structure is much higher than the value obtained from thermodynamic evaluation of the whole graphite structure. In fact, H2 is not able to freely enter between individual planes, and as a result, adsorption occurs mainly at the outer planes [28]. For the most promising results, the distance between individual planes should be enlarged and maintained at the level of about 0.6 nm [29]. From that point of view, the pillared graphene or, in general, spatial carbon-based materials, this group is of higher interest for the sake of hydrogen storage than graphite. The chemisorption requires a chemical reaction between carbon and hydrogen atoms. In the completely saturated state, carbon materials approach 1:1 stoichiometry of C to H. Such state can be achieved for fully hydrogenated graphene that is called graphane.
One of the important aspects of using carbon materials in terms of hydrogen storage is their large specific surface area, which has been indicated especially in the case of the use of activated carbon. According to the so-called “Chahine” rule[30], the amount of hydrogen stored can be proportional to the surface development. This means that one of the natural ways of research on hydrogen storage in carbon structures will be associated with the production of nanoporous spatial structures with a large surface, e.g., 3D graphene. Improvement of the gravimetric density of hydrogen stored by systems based on carbon-related materials is also connected with their structural and geometrical modifications or addition of compounds such as metallic catalyst.
4. Hydrogen Storage in Carbon Materials Not Based on Graphene Structures
Fullerenes (for example C60), allow to obtain up to 7.7 wt.% hydrogen. However, desorption of this gas takes place at high pressure and temperature (about 773 K and more, 50–120 bar). Fullerenes partially decompose during hydrogen release [31], leading to hydrogen contamination with volatile hydrocarbons [32]. Computer analyses of the decoration of fullerenes with metals show that these materials can reach gravimetric density even above 9 wt.% [33]. Experimental studies conducted with the use of light metals (which include lithium or sodium widely used in these applications) allow to obtain a value of this parameter above 4 wt.%, while ensuring the durability of the deposit. However, they require a hydrogenation temperature of 623 K at a pressure of 100 bar [34].
Both SWCNTs and MWCNTs allow for hydrogen storage in the range of up to 3.5 wt.% [35] and at pressures up to 140 bar. The maximum degree of nanotube hydrogenation depends on their diameter [36], but the control of this parameter is still a great technological challenge. The advantage of these materials, however, is the room temperature of work. To increase the possibility of hydrogen storage by CNTs, they can be decorated with metals, which has been proved by computer simulations for: titanium (up to 7.7 wt.%) [37], scandium (up to 9.8 wt.%) [38], aluminum (up to 6.15 wt.%) [39], or vanadium (up to 9.2 wt.%) [38]. However, most of the time, the amount of hydrogen stored in decorated CNTs often does not exceed 2 wt.% under experimental conditions [40]. Not only metals but also their oxides can be effectively used to decorate nanotubes. Vellingiri et al. [48],[49] in their publications described the achievement of storing 0.61 to 2.62 wt.% hydrogen in MWCNTs containing up to 9 wt.% SnO2 using a pressure of 5 bar and temperature of 373 K.
The use of high porosity carbon materials is primarily associated with the use of active carbon. This material can be obtained by treating various types of waste, mainly of plant origin. Obtaining the final product takes place in two stages, in which first the material is subjected to thermal decomposition of organic compounds (carbonization), and then high-temperature modification in an alkaline environment (most often KOH) with the participation of water vapor. The storage of hydrogen in active carbon is probably based solely on the principle of adsorption without the formation of a permanent atomic bond. As a result, the properties of the material under consideration strongly depend on its parameters, such as the size and morphology of the pores, and the specific surface area [51], [52]. The commercial use of this type of material is unfortunately limited due to the lack of technology capable of producing pores of a specific geometry and size.
Active carbon [51] obtained by means of biomass carbonization at various temperatures and modification environments, allows to obtain a gravimetric density of adsorbed hydrogen in the range of 3.99 to 5.05 wt.% for a temperature of 77 K and a pressure of 10 bar. This corresponds directly to the specific surface area of the material in the range from 2000 to 3100 m2/g and is inversely proportional to the size of the pores. Similarly, the porous carbon obtained [52] in the processes devoted to the carbonization of rayon fibers allows to develop the specific surface area up to the level of 3144 m2/g. For the material obtained in this way, the gravimetric density of adsorbed hydrogen at 77 K and 40 bar pressure ranged from 0.9 to 7.01 wt.%.
The most effective method of modifying the structure of porous carbon is the proper selection of intermediates in such a way as to obtain the desired chemical composition or structure after the hydrocarbonization and activation process. In the work of Blackenship et al. [53], the cellulose acetate used in the proposed work is characterized by a very high oxygen content (oxygen to carbon ratio = 0.83) in the chemical composition, allowing for its concentration in the final product to be controlled and, depending on its value, to analyze the degree of hydrogen sorption. Hydrogen sorbs on such material at a temperature of 77 K in the amount of 3.9 wt.% at a pressure of 1 bar to 8.9 wt.% at a pressure of 30 bar.
Storage of hydrogen in carbon materials such as nanotubes, fullerenes, or porous carbon, for the sake of automotive applications seems to still be a distant future. Conducted experimental researches still mainly result in low level of achieved gravimetric density. Moreover, there are still unwilling handling aspects to overcome such as: instability of those types of materials and their key parameters within the consecutive cycles of sorption/desorption, limitations related to the necessary usage of cryogenic temperatures or high pressures etc. Among the emerging solutions are those based on graphene.
5. Graphene-Assisted Hydrogen Storage
For hydrogen storage applications, graphene can be used directly as:
-
Basic material that takes part in sorption and desorption processes;
-
Scaffolding for metal catalysts allowing the spill-over processes to take place (sorption/desorption of graphene itself assisted by additional catalyst);
-
Material used to modify the existing solutions, e.g., composites based on graphene and metal hydrides.
The key to achieving a satisfactory sorption capacity of graphene-based systems is the synergistic effect of hydrogen chemisorption and physi-adsorption. By cyclically treating HSMG graphene sheets [54] with the hydrogen plasma action, cyclic changes in electrical resistance were obtained, corresponding to the graphene–graphane transformation [55]. In turn, the molecular modeling method showed that at temperatures interval close to ambient temperatures, hydrogenated graphene–graphane, exhibits hydrogenephilicity in contrast to hydrogenephobic graphene [56].
5.1. Plane Graphene and its Decorated Derivatives
Literature reports indicate that graphene and “graphene” materials in their pure form are not able to exceed the hydrogen storage barrier of a few percent by weight. To achieve this, very high pressure or low temperature are usually used. It should be noted, however, that nowadays reports are usually published covering mostly complex solutions (decorating, multi-component systems, etc.,) and not unmodified graphene. Of course, research is also conducted on the simple forms of graphene, e.g., graphene in the form of nanosheets or exfoliated graphene oxide (GO). The material with the potential to store hydrogen in this form was produced, among others, by Srinivas et. al. [57]. By using only the reduction of GO suspension with hydrazine, they obtained graphene sheets with a specific surface area of 640 m2/g. The experimental work carried out at a pressure of 10 bar showed the sorption capacity of this form of graphene at the level of 0.1 wt.% at room temperatures and even 1.2 wt.% for cryogenic temperatures. Kostoglou et al. [58] exfoliated graphene using microwave irradiation to obtain a material with a similar specific surface: about 630 m2/g. Such graphene structure at cryogenic temperatures and a pressure of 1 bar was characterized by a sorption capacity of 0.66 wt.%.
In the case of using multilayer graphene as a material for hydrogen storage, the obtained values of sorption capacity depend, inter alia, on the interlayer spacing. During oxidation, it is possible to control the distance between the graphene layers so that the hydrogen storage capacity is as high as possible. This effect can be observed when comparing the sorption capacity of GO and reduced-GO (rGO) by Rajuar et al. [59]. At room temperature, these materials were characterized by H2 adsorption of 1.9 wt.% and 1.34 wt.%, respectively, for a pressure of 80 bar and room temperature. The difference was related to the presence of oxygen-containing functional groups that separated the graphene layers more.
Many of the published studies in the field of possible use of graphene for hydrogen storage purposes include only calculations or preparation of simulations of possible structures based on this material. These analyses show, that depending on the decorator used, it is possible to achieve not only 5 wt.% when using calcium as a catalyst [60] but even 12 wt.% for lithium [61] or almost 14 wt.% for aluminum [62].
The main assumption behind the use of transition metal decorators deposited on graphene structures is related to the so-called “spill-over effect.” This process involves the initial dissociation of hydrogen in molecular form due to the presence of a catalyst dispersed on the surface. Then, migration of hydrogen to adjacent fragments of carbon material is possible, terminated by the diffusion of atomic hydrogen on the carbon support [63], [64]. These processes are particularly important in the case of research into materials for storing hydrogen directly at, or very close to, the room temperature. In this way, it is possible to use both physisorption and chemisorption processes to increase the total value of the sorption capacity. Of course, the obtained results will be undeniably influenced by both the fragmentation and uniformity of the catalyst dispersion, as evidenced by numerous publications conducted on various carbon materials, both nanotubes [65] and activated carbon [66].
The mechanism of spill-over processes is a subject of ongoing scientific discussions. There are still voices that its influence on hydrogen storage is exaggerated and the process itself may not take place at all [67]. These voices result, inter alia, from the unsuccessful verification of the research of Li et al. [68] by other research teams [69]. The spill-over effect was confirmed, among many others, by research conducted on the use of platinum as a catalyst. Zhou et al. [70] in his research on this subject used platinum nanoparticles (NPs) immobilized on a graphene composite and zeolitic imidazolate framework (ZIF) thanks to the facile liquid impregnation method. The obtained Pt@ZIF-8/GO material was characterized by a sorption capacity being 2.2 times higher than that of the starting material (ZIF-8) at a pressure of 10.0 bar and a temperature of 298 K.
One of the significant problems in decorating graphene [71] (but also other carbon structures [72]) with transition metals is therefore their agglomeration resulting from high cohesive forces. As a result, the decorated material is heterogeneous, and because of that issue, the uneven distribution of decorators results in a low sorption capacity. Introducing defects to the analyzed graphene structures (both vacancies and additional atoms) helps to eliminate this problem [73], [74].
The purpose of research on hydrogen storage in graphene structures is undoubtedly the broadly understood good of our planet’s humanity and ecology. Interestingly, among the proposed approaches to use graphene for H2 storage, you can find those that are also green at the stage of planning the experiment. Vinayan et al. [84] proposed to use the sun to produce a material with potential for hydrogen storage. In this case, the concentrated sunrays took part in the exfoliation of graphene while doping it with nitrogen and reducing the decorator, in this case, lead. The developed material was characterized by a sorption capacity of 4.3 wt.% (at 25 °C, 40 bar).
Nevertheless, these are still simulation values that are unlikely to be obtained in tests. On the other hand, many hopes are attached to various forms of graphene oxide and its reduced form. Moreover, the oxide can be decorated with nanoparticles, just like graphene. The experimental results currently indicate hydrogen adsorption in such structures reaching 5 wt.% [85], [86], [87].
The graphical representation of the undertaken approaches of graphene-decoration for the sake of hydrogen storage is presented in Figure 6.
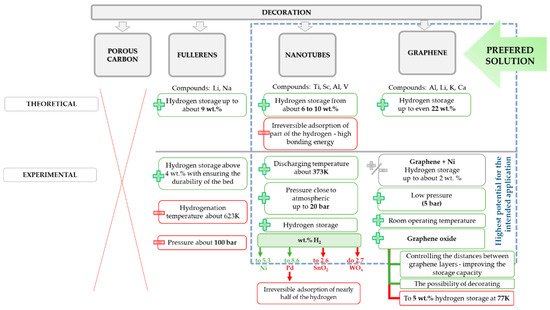
Figure 6. Summary of solutions for carbon-based materials decoration to upgrade their performance for the purpose of hydrogen storage.
5.2. Composites Containing Graphene
Zhang et al. [88] in their work dealt with the LiBH4 composite with porous fluorinated graphene structures with a specific surface area of about 220 m2/g. As a result of applying 20 wt.% multi-layers sheet-like structures, many important parameters in terms of target hydrogen storage applications have improved. The desorption temperature was lowered by about 120 °C in relation to the unmodified hydride, as well as the kinetics of hydrogen desorption was improved (activation energy decreased from 180.10 kJ/mol to 130.87 kJ/mol, enhanced cycling stability etc.). The developed material achieved a sorption capacity of 3.45 wt.% at 400 °C, which is 2.57 times more than that of unmodified LiBH4. The changes resulting from the use of the LiBH4 composite and the fluorinated graphene were related to easier recombination of hydrogen molecules on the surface of the material resulting from more reactive sites.
MgH2 + NH4Cl/graphene composite was proposed by Luo et al. [89] to reduce the temperature of hydrogen release to 437.8 K. This composite was obtained by mixing and grinding all components in a planetary mill in an argon atmosphere for the time period of 3 h. In this case, however, the graphene effect was small reduction of temperature by about 3.5 degree. The improvement of the functional properties by means of magnesium hydride by nearly 463 K was obtained primarily by appropriate selection of protonic and hydric H respectively from NH4Cl over MgH2. However, the presence of graphene had a positive effect on the purity of the recovered graphene, which was as high as 97.26%. The sorption capacity of the composites was 8.29 wt.% and 7.23 wt.% for the 5% and 10% graphene content, respectively.
Another interesting example of a composite with the potential for hydrogen storage is the combination of magnesium with graphene nanoplatelets (GNPs), i.e., structures containing up to 100 graphene layers [90]. By changing the parameters of the reactive grinding of graphite, from which GNPs with specific properties (size of platelets, specific surface area, etc.,) are obtained, it is possible to accelerate the hydrogen storage kinetics even by an order of magnitude. GNPs improve the migration of hydrogen between nearby Mg particles in various ways. In the case of large GNPs, bridges are formed between adjacent magnesium agglomerates, while small GNPs can create enclaves inside these agglomerates.
5.3. Spatial Graphene for Hydrogen Storage
Numerous approaches have been undertaken to move from the plane graphene to its spatial forms: foams or sponges, various scaffolds, pillared graphene, or 3D prints. Even hydrothermal methods can be introduced to achieve spatial forms of graphene [91]. In case of spatial graphene manufactured on scaffolds or templates acting as a support, very complex structures can be achieved by means of e.g., chemical vapor deposition or infiltration process in a liquid medium. Chen et. al. [92] produced flexible graphene foam on the nickel and copper support using template-directed chemical vapor deposition process. That structure was stiffened by poly(methyl methacrylate) prior to etching the metal with hot FeCl3 solution. For the 3D graphene, the polymer layer was removed with hot acetone. Depending on the type and supportive metal foam the graphene foam with surface area up to 850 m2/g and porosity of even 99.7% was achieved. The contamination of the final product with residues of scaffold is a problematic case of methods based on various templates. Moreover, the removal of the metal scaffold is so aggressive that the deformation of desired structures can occur.
Graphene foams with a specific surface area reaching even over 1250 m2/g can be obtained for example as a result of combustion of sodium ethoxide. Lyth et al. [95] reported the achievement of 2.1 wt.% of hydrogen storage (77 K and 10 bar) for such surface development.
Structures known as graphene sponges are obtained, for example, using the technology of freeze drying [58] and a GO suspension treated with hydroiodic acid. As a result of the adopted production method, spatial graphene was obtained with a specific surface area of approximately 350 m2/g, which at a temperature of 77 K and a pressure of 1 bar obtained a sorption capacity of 0.85 wt.%.
Big family of methods used for the sake of spatial graphene synthesis involves various types of pillars introduced between the graphene planes. Such modification can be based on long-chain polyamides bonding to graphene via amidation [97], polyethyleneimine [98], metal particles [99], or even other carbon-based material as fullerenes [100] or carbon nanotubes [101]. Researches conducted by Banda et al. [102] proves that number of pillars has major effect on the properties of spatial graphene. In case of sparsely filling, the gallery structures are preserved, and this facilitates the accessibility to new active sites.
One of the novel approaches involve 2-step procedure based on use of hydrazine not as a reducing agent but as a compound that cross-links the graphene oxide suspension. This original concept was first confirmed experimentally on a model material-monolayer, quasi-monocrystalline metallurgical graphene HSMG, from which a three-layer nitrogen-pillared sandwich was made using local pre-oxidation sites [103]. In industrial scaling, GO was used as a mass substrate. The first step involves the use of an oxygen-containing defects (C=O, –COOH, and –OH groups) substitution reaction with hydrazine carried out at 328 K. As a result, the graphene flakes are pillared with N-N bridges. In the second step, the complete removal of the remaining oxygen groups takes place as a result of reduction conducted in 973 K in hydrogen overpressure.
Computer simulations on graphene pillared with fullerenes show that at cryo temperatures, the hydrogen uptake of such structures may reach 4.0 wt.% at 1 bar and 10.3 wt.% at 100 bar [104], when the free surface area is over 1755 m2/g. In the case of additional decoration of such complex structures with lithium, estimated hydrogen storage capacity may rise to 9.1 wt.% at 77 K and 1 bar [105].
Obviously, the idea of decoration of spatial forms of graphene is under careful investigation. One of the possible approaches involve electrostatic assembling and poly (methyl methacrylate) (PMMA) template [106]. GO-COOH dispersion self-assemble on the surface of positively charged microspheres made of PMMA, and after the addition of NiCl2 calcinated in 600 °C in nitrogen atmosphere to remove PMMA. Such material with Ni nanoparticles located on the graphene porous structures shows hydrogen storage capacity of 1.95 and 4.22 wt.% respectively in 298 K and 77 K under a pressure of 5 bar.
An attempt was made and described also with HSMG metallurgical graphene decorated with SiC solid particles directly in the phase of nucleation and growth on the surface of liquid copper [25]. The preferential, heterogeneous nucleation of the first and subsequent graphene layers on SiC micro and nanoparticles was demonstrated. These studies were carried out with a view to producing a decorated graphene substrate for the production of spiral, spaced spatial nanostructures [108].
Although the amount of hydrogen stored in graphene based materials is still lower than expected, a wide range of investigated solutions (involving different ways of spatialization and pillaring or decoration with various compounds) give hope of upcoming breakthrough in the field of introduction of these materials in green, sustainable energy systems.
6. Future Perspectives
Hydrogen as an energy carrier and a potential fuel is a sustainable source of energy, that can be produced from a variety of different sources, both renewable and non-renewable. H2 might serve as a promising “fuel of the future” in many different fields of industry, mainly because of its benefits over traditional energy sources in social, economic, and also environmental aspects. Because of that, for the past decades, many investigations have been made primarily to find a way to efficiently and cost-effectively store and transport hydrogen to be used as a potential fuel.
However nowadays, scientists list several technological barriers that need to be removed in order to finish using the carbon-based energy and focus on the systems based on more green resources like hydrogen. Among them, important are the aspects of cost-efficient and sustainable H2 production and supply, its storage for not only stationary but also mobile applications, and further costs of hydrogen used as a fuel. All of these aspects directly depend on many economic factors, the development rate of technologies that will be based on hydrogen, as well as the urgent need to reduce the greenhouse gas reduction caused in particular by the extensive use of fossil fuels. Taking these into consideration it must be noticed that the main challenge before making hydrogen a common source of energy is its storage.
Over the past years, many various methods have been proposed to fulfill the needs of sufficient H2 storage. Among them, we could list methods based on chemical or physical interactions (storage of compressed gas, adsorption/adsorption, use of materials like carbon-based structures). However, the true challenge for development of hydrogen adsorbing materials is to ensure the light-weight, light-carrier materials that have a sufficient amount of bonding sites. Hence, a significant amount of interest of scientists is focused on the use of materials like graphite, zeolites, carbon nanotubes, metal organic frameworks, and many others. The graphical representation of advantages and disadvantages of different carbon-based materials planned to use for hydrogen storage purposes is depicted in the Figure 7.
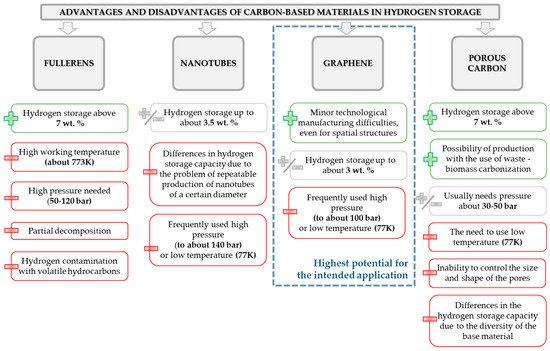
Figure 7. Summary of the advantages and disadvantages of frequently used carbon-based materials intended to use for hydrogen storage.
A significant progress has been made in the development of materials for hydrogen storage of which action will be focused on desorption and absorption properties of nanoscale materials with high specific surface area. Among the proposed solution, one of the promising ways to fulfill the needs of efficient hydrogen storage is the use of graphene-based structures. Authors conclude that spatial graphene decorated not only on the outer surfaces but also inside the porosities, may be a solution for hydrogen storage at ambient temperatures and pressures of at most 5 bars.
The strategic issues for the industrial scaling of hydrogen storage systems based on materials using graphene are:
-
Working of the technology of mass production of the substrates—GO and/or rGO with a high degree of exfoliation and purity at the level of at least 99.99% C;
-
Development of chemically durable and mechanically resistant, spatially pillared, nanoporous 3D graphene structures with an active surface of at least 1000 m2/g;
-
Selection of effective “spill-over” catalysts with a range of reversible reactions with hydrogen identical to the reversible reaction of graphene–graphene;
-
Development of a technology of spatial decoration of nanoporous 3D graphene structures with nanoparticles of optimal “spill-over” catalysts.
Such graphene-based material may be a key to unlocking the global usage of hydrogen in mobile applications e.g., automotive industry.
This entry is adapted from the peer-reviewed paper 10.3390/ma14102499
References
- Ibrahim Dincer; Environmental and sustainability aspects of hydrogen and fuel cell systems. International Journal of Energy Research 2006, 31, 29-55, 10.1002/er.1226.
- N Muradov; T Veziroglu; “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. International Journal of Hydrogen Energy 2008, 33, 6804-6839, 10.1016/j.ijhydene.2008.08.054.
- J.O. Abe; A.P.I. Popoola; Emmanuel Ajenifuja; Olawale Popoola; Hydrogen energy, economy and storage: Review and recommendation. International Journal of Hydrogen Energy 2019, 44, 15072-15086, 10.1016/j.ijhydene.2019.04.068.
- Ibrahim Dincer; Canan Acar; Review and evaluation of hydrogen production methods for better sustainability. International Journal of Hydrogen Energy 2015, 40, 11094-11111, 10.1016/j.ijhydene.2014.12.035.
- Ming He; Yu Zhang; Linghui Gong; Yuan Zhou; Xiufang Song; Weiping Zhu; Meimei Zhang; Zhao Zhang; Bibliometrical analysis of hydrogen storage. International Journal of Hydrogen Energy 2019, 44, 28206-28226, 10.1016/j.ijhydene.2019.07.014.
- M. Ball; M. Wietschel; The future of hydrogen – opportunities and challenges. International Journal of Hydrogen Energy 2009, 34, 615-627, 10.1016/j.ijhydene.2008.11.014.
- Sanjay Kumar; Ankur Jain; T. Ichikawa; Y. Kojima; G.K. Dey; Development of vanadium based hydrogen storage material: A review. Renewable and Sustainable Energy Reviews 2017, 72, 791-800, 10.1016/j.rser.2017.01.063.
- H. Barthelemy; M. Weber; F. Barbier; Hydrogen storage: Recent improvements and industrial perspectives. International Journal of Hydrogen Energy 2017, 42, 7254-7262, 10.1016/j.ijhydene.2016.03.178.
- Sunita Satyapal; John Petrovic; Carole Read; George Thomas; Grace Ordaz; The U.S. Department of Energy's National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catalysis Today 2007, 120, 246-256, 10.1016/j.cattod.2006.09.022.
- Fan Zhang; Pengcheng Zhao; Meng Niu; Jon Maddy; The survey of key technologies in hydrogen energy storage. International Journal of Hydrogen Energy 2016, 41, 14535-14552, 10.1016/j.ijhydene.2016.05.293.
- P.P. Edwards; V.L. Kuznetsov; W.I.F. David; N.P. Brandon; Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356-4362, 10.1016/j.enpol.2008.09.036.
- Andreas Züttel; Hydrogen storage methods. The Science of Nature 2004, 91, 157-172, 10.1007/s00114-004-0516-x.
- Sunita Sharma; Sib Krishna Ghoshal; Hydrogen the future transportation fuel: From production to applications. Renewable and Sustainable Energy Reviews 2015, 43, 1151-1158, 10.1016/j.rser.2014.11.093.
- L. Barreto; A. Makihira; K. Riahi; The hydrogen economy in the 21st century: a sustainable development scenario. International Journal of Hydrogen Energy 2003, 28, 267-284, 10.1016/s0360-3199(02)00074-5.
- T. Nejat Veziroğlu; Sümer Şahi˙n; 21st Century’s energy: Hydrogen energy system. Energy Conversion and Management 2008, 49, 1820-1831, 10.1016/j.enconman.2007.08.015.
- Mustafa Balat; Potential importance of hydrogen as a future solution to environmental and transportation problems. International Journal of Hydrogen Energy 2008, 33, 4013-4029, 10.1016/j.ijhydene.2008.05.047.
- G.W. Crabtree; M.S. Dresselhaus; The Hydrogen Fuel Alternative. MRS Bulletin 2008, 33, 421-428, 10.1557/mrs2008.84.
- K. S. Novoselov; A. K. Geim; S. V. Morozov; D. Jiang; Y. Zhang; S. V. Dubonos; I. V. Grigorieva; A. A. Firsov; Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666-669, .
- D. D. L. Chung; Review Graphite. Journal of Materials Science 2002, 37, 1475-1489, 10.1023/A:1014915307738.
- Kevin F. McCarty; Peter J. Feibelman; Elena Loginova; Norman C. Bartelt; Kinetics and thermodynamics of carbon segregation and graphene growth on Ru(0001). Carbon 2009, 47, 1806-1813, 10.1016/j.carbon.2009.03.004.
- Pei Zhao; Akihito Kumamoto; Sungjin Kim; Xiao Chen; Bo Hou; Shohei Chiashi; Erik Einarsson; Yuichi Ikuhara; Shigeo Maruyama; Self-Limiting Chemical Vapor Deposition Growth of Monolayer Graphene from Ethanol. The Journal of Physical Chemistry C 2013, 117, 10755-10763, 10.1021/jp400996s.
- Jeonghyun Hwang; Moonkyung Kim; Virgil B. Shields; Michael G. Spencer; CVD growth of SiC on sapphire substrate and graphene formation from the epitaxial SiC. Journal of Crystal Growth 2013, 366, 26-30, 10.1016/j.jcrysgro.2012.12.136.
- K. Wang; G. Tai; K. H. Wong; Shu Ping Lau; W. Guo; Ni induced few-layer graphene growth at low temperature by pulsed laser deposition. AIP Advances 2011, 1, 22141, 10.1063/1.3602855.
- Patent No. EP 2865646
- Piotr Kula; Łukasz Kaczmarek; Piotr Zawadzki; Lukasz Kolodziejczyk; Witold Szymański; Piotr Niedzielski; Robert Pietrasik; Konrad Dybowski; Dariusz Kazimierski; Dorota Nowak; et al. Functionality of graphene as a result of its heterogenic growth on SiC nanoparticles on the basis of reversible hydrogen storage. International Journal of Hydrogen Energy 2014, 39, 19662-19671, 10.1016/j.ijhydene.2014.09.157.
- N.I. Zaaba; K.L. Foo; U. Hashim; S.J. Tan; Wei Wen Liu; C.H. Voon; Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Engineering 2017, 184, 469-477, 10.1016/j.proeng.2017.04.118.
- Seul-Yi Lee; Soo-Jin Park; Comprehensive review on synthesis and adsorption behaviors of graphene-based materials. Carbon Letters 2012, 13, 73-87, 10.5714/cl.2012.13.2.073.
- J. S. Arellano; L. M. Molina; A. Rubio; J. A. Alonso; Density Functional Study of adsorption of molecular hydrogen on graphene layers. J. Chem. Phys 2000, 112, 8114, .
- Wenhui Yuan; Baoqing Li; Li Li; A green synthetic approach to graphene nanosheets for hydrogen adsorption. Applied Surface Science 2011, 257, 10183-10187, 10.1016/j.apsusc.2011.07.015.
- Barbara Panella; Michael Hirscher; Siegmar Roth; Hydrogen adsorption in different carbon nanostructures. Carbon 2005, 43, 2209-2214, 10.1016/j.carbon.2005.03.037.
- Serhiy M. Luzan; Yury O. Tsybin; Alexandr V. Talyzin; Reaction of C60 with Hydrogen Gas: In Situ Monitoring and Pathways. The Journal of Physical Chemistry C 2011, 115, 11484-11492, 10.1021/jp202715g.
- A.V. Talyzin; Y.M. Shulga; A. Jacob; Comparative study of hydrofullerides C 60 H x synthesized by direct and catalytic hydrogenation. Applied Physics A 2004, 78, 1005-1010, 10.1007/s00339-003-2422-z.
- Yuanchang Li; Gang Zhou; Jia Li; Bin-Ling Gu; Wenhui Duan; Alkali-Metal-Doped B80 as High-Capacity Hydrogen Storage Media. The Journal of Physical Chemistry C 2008, 112, 19268-19271, 10.1021/jp807156g.
- Gerasimos E. Ioannatos; Xenophon E. Verykios; H2 storage on single- and multi-walled carbon nanotubes. International Journal of Hydrogen Energy 2010, 35, 622-628, 10.1016/j.ijhydene.2009.11.029.
- Anton Nikitin; Xiaolin Li; Zhiyong Zhang; Hirohito Ogasawara; Hongjie Dai; Anders Nilsson; Hydrogen Storage in Carbon Nanotubes through the Formation of Stable C−H Bonds. Nano Letters 2007, 8, 162-167, 10.1021/nl072325k.
- T. Yildirim; S. Ciraci; Titanium-Decorated Carbon Nanotubes as a Potential High-Capacity Hydrogen Storage Medium. Physical Review Letters 2005, 94, 175501-175501, 10.1103/physrevlett.94.175501.
- E. Durgun; S. Ciraci; T. Yildirim; Functionalization of carbon-based nanostructures with light transition-metal atoms for hydrogen storage. Physical Review B 2008, 77, 085405, 10.1103/physrevb.77.085405.
- S. Seenithurai; R. Kodi Pandyan; S. Vinodh Kumar; C. Saranya; M. Mahendran; Al-decorated carbon nanotube as the molecular hydrogen storage medium. International Journal of Hydrogen Energy 2014, 39, 11990-11998, 10.1016/j.ijhydene.2014.05.184.
- Larijani, M.M.; Safa, S.; Increase of hydrogen storage capacity of CNTs by using Transition metal, Metal oxide-CNT nanocomposites. Acta Phys. Pol. A 2014, 126, 732-735, .
- Joseph A. Teprovich; Douglas A. Knight; Brent Peters; Ragaiy Zidan; Comparative study of reversible hydrogen storage in alkali-doped fulleranes. Journal of Alloys and Compounds 2013, 580, S364-S367, 10.1016/j.jallcom.2013.02.024.
- A Leelamohanareddy; Hydrogen adsorption properties of single-walled carbon nanotube—Nanocrystalline platinum composites. International Journal of Hydrogen Energy 2008, 33, 1028-1034, 10.1016/j.ijhydene.2007.11.005.
- Chun-Chen Yang; Yingjeng James Li; Wei-Huang Chen; Electrochemical hydrogen storage behavior of single-walled carbon nanotubes (SWCNTs) coated with Ni nanoparticles. International Journal of Hydrogen Energy 2010, 35, 2336-2343, 10.1016/j.ijhydene.2010.01.007.
- M. Mehrabi; P. Parvin; A. Reyhani; S.Z. Mortazavi; Hybrid laser ablation and chemical reduction to synthesize Ni/Pd nanoparticles decorated multi-wall carbon nanotubes for effective enhancement of hydrogen storage. International Journal of Hydrogen Energy 2018, 43, 12211-12221, 10.1016/j.ijhydene.2018.04.144.
- † Hyun-Seok Kim; ‡ Ho Lee; † Kyu-Sung Han; § Jin-Ho Kim; † Min-Sang Song; † Min-Sik Park; † And Jai-Young Lee; † Jeung-Ku Kang; Hydrogen Storage in Ni Nanoparticle-Dispersed Multiwalled Carbon Nanotubes. The Journal of Physical Chemistry B 2005, 109, 8983-8986, 10.1021/jp044727b.
- Songül Kaskun; Muhammet Kayfeci; The synthesized nickel-doped multi-walled carbon nanotubes for hydrogen storage under moderate pressures. International Journal of Hydrogen Energy 2018, 43, 10773-10778, 10.1016/j.ijhydene.2018.01.084.
- Y. Suttisawat; P. Rangsunvigit; B. Kitiyanan; M. Williams; P. Ndungu; M.V. Lototskyy; A. Nechaev; V. Linkov; S. Kulprathipanja; Investigation of hydrogen storage capacity of multi-walled carbon nanotubes deposited with Pd or V. International Journal of Hydrogen Energy 2009, 34, 6669-6675, 10.1016/j.ijhydene.2009.06.063.
- Sami-Ullah Rather; Renju Zacharia; Sang Woon Hwang; Mehraj-Ud-Din Naik; Kee Suk Nahm; Hydrogen uptake of palladium-embedded MWCNTs produced by impregnation and condensed phase reduction method. Chemical Physics Letters 2007, 441, 261-267, 10.1016/j.cplett.2007.05.006.
- Lathapriya Vellingiri; Karthigeyan Annamalai; Ramamurthi Kandasamy; Iyakutti Kombiah; Characterization and hydrogen storage properties of SnO2 functionalized MWCNT nanocomposites. International Journal of Hydrogen Energy 2018, 43, 10396-10409, 10.1016/j.ijhydene.2018.04.120.
- Lathapriya Vellingiri; Karthigeyan Annamalai; Ramamurthi Kandasamy; Iyakutti Kombiah; Synthesis and characterization of MWCNT impregnated with different loadings of SnO2 nanoparticles for hydrogen storage applications. International Journal of Hydrogen Energy 2018, 43, 848-860, 10.1016/j.ijhydene.2017.11.023.
- D. Silambarasan; V. J. Surya; V. Vasu; K. Iyakutti; Single Walled Carbon Nanotube–Metal Oxide Nanocomposites for Reversible and Reproducible Storage of Hydrogen. ACS Applied Materials & Interfaces 2013, 5, 11419-11426, 10.1021/am403662t.
- Fangyi Cheng; Jing Liang; Jianzhi Zhao; Zhanliang Tao; Jun Chen; Biomass Waste-Derived Microporous Carbons with Controlled Texture and Enhanced Hydrogen Uptake. Chemistry of Materials 2008, 20, 1889-1895, 10.1021/cm702816x.
- Yan Li; Donglin Zhao; Yuntao Wang; Risheng Xue; Zengmin Shen; Xingguo Li; The mechanism of hydrogen storage in carbon materials. International Journal of Hydrogen Energy 2007, 32, 2513-2517, 10.1016/j.ijhydene.2006.11.010.
- Troy Scott Blankenship Ii; Norah Balahmar; Robert Mokaya; Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nature Communications 2017, 8, 1-12, 10.1038/s41467-017-01633-x.
- P. Kula; W. Szymański; Ł. Kołodziejczyk; Radomir Atraszkiewicz; K. Dybowski; Jacek Grabarczyk; R. Pietrasik; P. Niedzielski; Ł. Kaczmarek; M. Cłapa; et al. High Strength Metallurgical Graphene – Mechanisms of Growth and Properties / Grafen Metalurgiczny O Wysokiej Wytrzymałości – Mechanizmy Wzrostu I Właściwości. Archives of Metallurgy and Materials 2015, 60, 2535-2542, 10.1515/amm-2015-0273.
- Piotr Kula; Witold Szymanski; Lukasz Kolodziejczyk; Radomir Atraszkiewicz; Jacek Grabarczyk; Marian Clapa; Lukasz Kaczmarek; Anna Jedrzejczak; Piotr Niedzielski; High strength metallurgical graphene for hydrogen storage nanocomposites. Vacuum 2016, 129, 79-85, 10.1016/j.vacuum.2016.04.017.
- Ł. Kaczmarek; T. Warga; P. Zawadzki; M. Makowicz; B. Bucholc; P. Kula; The influence of the hydrogenation degree on selected properties of graphene as a material for reversible H2 storage. International Journal of Hydrogen Energy 2019, 44, 23149-23159, 10.1016/j.ijhydene.2019.06.007.
- G. Srinivas; Yanwu Zhu; Richard Piner; Neal Skipper; Mark Ellerby; Rod Ruoff; Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630-635, 10.1016/j.carbon.2009.10.003.
- Nikolaos Kostoglou; Vasilios Tzitzios; Athanassios G. Kontos; Konstantinos Giannakopoulos; Christos Tampaxis; Aggeliki Papavasiliou; Georgia Charalambopoulou; Theodore Steriotis; Yuanqing Li; Kin Liao; et al. Synthesis of nanoporous graphene oxide adsorbents by freeze-drying or microwave radiation: Characterization and hydrogen storage properties. International Journal of Hydrogen Energy 2015, 40, 6844-6852, 10.1016/j.ijhydene.2015.03.053.
- Rajveer Singh Rajaura; Subodh Srivastava; Vinay Sharma; P.K. Sharma; C. Lal; Mangej Singh; H.S. Palsania; Y.K. Vijay; Role of interlayer spacing and functional group on the hydrogen storage properties of graphene oxide and reduced graphene oxide. International Journal of Hydrogen Energy 2016, 41, 9454-9461, 10.1016/j.ijhydene.2016.04.115.
- Vinayan Bhagavathi Parambhath; Rupali Nagar; S. Ramaprabhu; Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826-7833, 10.1021/la301232r.
- Lai-Peng Ma; Zhong-Shuai Wu; Jing Li; Er-Dong Wu; Wen-Cai Ren; Hui-Ming Cheng; Hydrogen adsorption behavior of graphene above critical temperature. International Journal of Hydrogen Energy 2009, 34, 2329-2332, 10.1016/j.ijhydene.2008.12.079.
- Anupama Ghosh; K. S. Subrahmanyam; Katla Sai Krishna; Sudipta Datta; A. Govindaraj; Swapan K. Pati; C. N. R. Rao; Uptake of H2 and CO2 by Graphene. The Journal of Physical Chemistry C 2008, 112, 15704-15707, 10.1021/jp805802w.
- Cheng-Yu Wang; Cheng-Wei Chang; Yi-Ju Wu; Angela D Lueking; Observation and simulation of hydrogen storage via spillover. Current Opinion in Chemical Engineering 2018, 21, 116-121, 10.1016/j.coche.2018.10.005.
- Lifeng Wang; Jr Anthony J. Lachawiec; Ralph T. Yang; Nanostructured adsorbents for hydrogen storage at ambient temperature: high-pressure measurements and factors influencing hydrogen spillover. RSC Advances 2013, 3, 23935-23952, 10.1039/c3ra44216k.
- Renju Zacharia; Sami-Ullah Rather; Sang Woon Hwang; Kee Suk Nahm; Spillover of physisorbed hydrogen from sputter-deposited arrays of platinum nanoparticles to multi-walled carbon nanotubes. Chemical Physics Letters 2007, 434, 286-291, 10.1016/j.cplett.2006.12.022.
- Zhen Geng; Dabin Wang; Cunman Zhang; Xiangyang Zhou; Haifeng Xin; Xupeng Liu; Mei Cai; Spillover enhanced hydrogen uptake of Pt/Pd doped corncob-derived activated carbon with ultra-high surface area at high pressure. International Journal of Hydrogen Energy 2014, 39, 13643-13649, 10.1016/j.ijhydene.2014.02.065.
- Vatsal Jain; Balasubramanian Kandasubramanian; Functionalized graphene materials for hydrogen storage. Journal of Materials Science 2019, 55, 1865-1903, 10.1007/s10853-019-04150-y.
- Yingwei Li; Ralph T. Yang; Hydrogen Storage in Metal−Organic Frameworks by Bridged Hydrogen Spillover. Journal of the American Chemical Society 2006, 128, 8136-8137, 10.1021/ja061681m.
- Renato Campesi; Fermín Cuevas; Michel Latroche; Michael Hirscher; Hydrogen spillover measurements of unbridged and bridged metal–organic frameworks—revisited. Physical Chemistry Chemical Physics 2010, 12, 10457-10459, 10.1039/c0cp00037j.
- Hu Zhou; Jun Zhang; Jian Zhang; Xiufen Yan; Xiaoping Shen; Aihua Yuan; High-capacity room-temperature hydrogen storage of zeolitic imidazolate framework/graphene oxide promoted by platinum metal catalyst. International Journal of Hydrogen Energy 2015, 40, 12275-12285, 10.1016/j.ijhydene.2015.05.199.
- Kevin T. Chan; J. B. Neaton; Marvin L. Cohen; First-principles study of metal adatom adsorption on graphene. Physical Review B 2008, 77, 313-318, 10.1103/physrevb.77.235430.
- Qiang Sun; † Qian Wang; Puru Jena; Yoshiyuki Kawazoe‡; Clustering of Ti on a C60Surface and Its Effect on Hydrogen Storage. Journal of the American Chemical Society 2005, 127, 14582-14583, 10.1021/ja0550125.
- Woon Ih Choi; Seung-Hoon Jhi; Kwiseon Kim; Yong-Hyun Kim; Divacancy-nitrogen-assisted transition metal dispersion and hydrogen adsorption in defective graphene: A first-principles study. Physical Review B 2010, 81, 085441, 10.1103/physrevb.81.085441.
- Shibing Chu; Leibo Hu; Xianru Hu; Mingkun Yang; Jianbo Deng; Titanium-embedded graphene as high-capacity hydrogen-storage media. International Journal of Hydrogen Energy 2011, 36, 12324-12328, 10.1016/j.ijhydene.2011.07.015.
- Zahra Gohari Bajestani; Alp Yürüm; Significant improvement in the hydrogen storage capacity of a reduced graphene oxide/TiO 2 nanocomposite by chemical bonding of Ti–O–C. RSC Advances 2016, 6, 32831-32838, 10.1039/C6RA00944A.
- Mohammad Choucair; Philippe Mauron; Versatile preparation of graphene-based nanocomposites and their hydrogen adsorption. International Journal of Hydrogen Energy 2015, 40, 6158-6164, 10.1016/j.ijhydene.2015.03.065.
- Won G. Hong; Byung Hoon Kim; Sang Moon Lee; Han Young Yu; Yong Ju Yun; Yongseok Jun; Jin Bae Lee; Hae Jin Kim; Agent-free synthesis of graphene oxide/transition metal oxide composites and its application for hydrogen storage. International Journal of Hydrogen Energy 2012, 37, 7594-7599, 10.1016/j.ijhydene.2012.02.010.
- A. Gotzias; E. Tylianakis; G. Froudakis; Th. Steriotis; Theoretical study of hydrogen adsorption in oxygen functionalized carbon slit pores. Microporous and Mesoporous Materials 2012, 154, 38-44, 10.1016/j.micromeso.2011.10.011.
- D. Giasafaki; A. Bourlinos; G. Charalambopoulou; A. Stubos; Th. Steriotis; Synthesis and characterisation of nanoporous carbon–metal composites for hydrogen storage. Microporous and Mesoporous Materials 2012, 154, 74-81, 10.1016/j.micromeso.2011.11.011.
- Tsui-Yun Chung; Cheng-Si Tsao; Hui-Ping Tseng; Chien-Hung Chen; Ming-Sheng Yu; Effects of oxygen functional groups on the enhancement of the hydrogen spillover of Pd-doped activated carbon. Journal of Colloid and Interface Science 2015, 441, 98-105, 10.1016/j.jcis.2014.10.062.
- Zhifen Luo; Xiaoli Fan; Rui Pan; Yurong An; A first-principles study of Sc-decorated graphene with pyridinic-N defects for hydrogen storage. International Journal of Hydrogen Energy 2017, 42, 3106-3113, 10.1016/j.ijhydene.2016.11.039.
- Gyubong Kim; Seung-Hoon Jhi; Noejung Park; Steven G. Louie; Marvin L. Cohen; Optimization of metal dispersion in doped graphitic materials for hydrogen storage. Physical Review B 2008, 78, 085408, 10.1103/physrevb.78.085408.
- Omar Faye; Ubong Eduok; Jerzy Szpunar; Barbara Szpunar; Almoustapha Samoura; Aboubaker Beye; Hydrogen storage on bare Cu atom and Cu-functionalized boron-doped graphene: A first principles study. International Journal of Hydrogen Energy 2017, 42, 4233-4243, 10.1016/j.ijhydene.2016.10.031.
- Bhaghavathi Parambath Vinayan; Rupali Nagar; S. Ramaprabhu; Solar light assisted green synthesis of palladium nanoparticle decorated nitrogen doped graphene for hydrogen storage application. Journal of Materials Chemistry A 2013, 1, 11192-11199, 10.1039/c3ta12016c.
- N. Ismail; M. Madian; M. Samy El-Shall; Reduced graphene oxide doped with Ni/Pd nanoparticles for hydrogen storage application. Journal of Industrial and Engineering Chemistry 2015, 30, 328-335, 10.1016/j.jiec.2015.06.002.
- Seyed Hamed Aboutalebi; Sima Aminorroaya-Yamini; Ivan Nevirkovets; Konstantin Konstantinov; Hua Kun Liu; Enhanced Hydrogen Storage in Graphene Oxide-MWCNTs Composite at Room Temperature. Advanced Energy Materials 2012, 2, 1439-1446, 10.1002/aenm.201200154.
- Byung Hoon Kim; Won G. Hong; Han Young Yu; Young-Kyu Han; Sang Moon Lee; Sung Jin Chang; Hoi Ri Moon; Yongseok Jun; Hae Jin Kim; Thermally modulated multilayered graphene oxide for hydrogen storage. Physical Chemistry Chemical Physics 2011, 14, 1480-1484, 10.1039/c2cp23683d.
- Wei Zhang; Guang Xu; Lingjuan Chen; Shuyi Pan; Xu Jing; Jiasheng Wang; Shumin Han; Enhanced hydrogen storage performances of LiBH4 modified with three-dimensional porous fluorinated graphene. International Journal of Hydrogen Energy 2017, 42, 15262-15270, 10.1016/j.ijhydene.2017.05.018.
- Bosang Luo; Zhendong Yao; Xuezhang Xiao; Zhouming Hang; Fulei Jiang; Meijia Liu; Lixin Chen; Hydrogen desorption from MgH2+NH4Cl/graphene composites at low temperatures. Materials Chemistry and Physics 2021, 263, 124342, 10.1016/j.matchemphys.2021.124342.
- Efrat Ruse; Matat Buzaglo; Ilan Pri-Bar; Liran Shunak; Roey Nadiv; Svetlana Pevzner; Orit Siton-Mendelson; Vladimir M. Skripnyuk; Eugen Rabkin; Oren Regev; et al. Hydrogen storage kinetics: The graphene nanoplatelet size effect. Carbon 2018, 130, 369-376, 10.1016/j.carbon.2018.01.012.
- Yuxi Xu; Kaixuan Sheng; Chun Li; Gaoquan Shi; Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324-4330, 10.1021/nn101187z.
- Zongping Chen; Wencai Ren; Libo Gao; Bilu Liu; Songfeng Pei; Hui-Ming Cheng; Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nature Materials 2011, 10, 424-428, 10.1038/nmat3001.
- Junyi Ji; Jilei Liu; Linfei Lai; Xin Zhao; Yongda Zhen; Jianyi Lin; Yanwu Zhu; Hengxing Ji; Li Li Zhang; Rodney S. Ruoff; et al. In Situ Activation of Nitrogen-Doped Graphene Anchored on Graphite Foam for a High-Capacity Anode. ACS Nano 2015, 9, 8609-8616, 10.1021/acsnano.5b03888.
- Kaisheng Xia; Xiangle Tian; Shunxin Fei; Kun You; Hierarchical porous graphene-based carbons prepared by carbon dioxide activation and their gas adsorption properties. International Journal of Hydrogen Energy 2014, 39, 11047-11054, 10.1016/j.ijhydene.2014.05.059.
- Stephen Matthew Lyth; Huaiyu Shao; Jianfeng Liu; Kazunari Sasaki; Etsuo Akiba; Hydrogen adsorption on graphene foam synthesized by combustion of sodium ethoxide. International Journal of Hydrogen Energy 2014, 39, 376-380, 10.1016/j.ijhydene.2013.10.044.
- Haiyan Sun; Zhen Xu; Chao Gao; Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Advanced Materials 2013, 25, 2554-2560, 10.1002/adma.201204576.
- Binghu Wang; Yong Qin; Wensheng Tan; Yongxin Tao; Yong Kong; Smartly designed 3D N-doped mesoporous graphene for high-performance supercapacitor electrodes. Electrochimica Acta 2017, 241, 1-9, 10.1016/j.electacta.2017.04.120.
- Zhu-Yin Sui; Yi Cui; Jian-Hua Zhu; Bao-Hang Han; Preparation of Three-Dimensional Graphene Oxide–Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Applied Materials & Interfaces 2013, 5, 9172-9179, 10.1021/am402661t.
- Chengbin Liu; Ke Wang; Shenglian Luo; Yanhong Tang; Liuyun Chen; Direct Electrodeposition of Graphene Enabling the One-Step Synthesis of Graphene-Metal Nanocomposite Films. Small 2011, 7, 1203-1206, 10.1002/smll.201002340.
- Vincent C. Tung; Jen-Hsien Huang; Ian Tevis; Franklin Kim; Jaemyung Kim; Chih-Wei Chu; Samuel I. Stupp; Jiaxing Huang; Surfactant-Free Water-Processable Photoconductive All-Carbon Composite. Journal of the American Chemical Society 2011, 133, 4940-4947, 10.1021/ja1103734.
- Hesam Amiri; Jamshid Aghazadeh Mohandesi; Pirooz Marashi; Tensile properties of pillared graphene block. Materials Science and Engineering: B 2020, 257, 114557, 10.1016/j.mseb.2020.114557.
- Harish Banda; Sandy Périé; Barbara Daffos; Pierre-Louis Taberna; Lionel Dubois; Olivier Crosnier; Patrice Simon; Daniel Lee; Gaël De Paëpe; Florence Duclairoir; et al. Sparsely Pillared Graphene Materials for High-Performance Supercapacitors: Improving Ion Transport and Storage Capacity. ACS Nano 2019, 13, 1443-1453, 10.1021/acsnano.8b07102.
- Lukasz Kaczmarek; Piotr Kula; Tomasz Warga; Lukasz Kolodziejczyk; Petr Louda; Karolína Borůvková; Piotr Niedzielski; Witold Szymanski; Lukáš Voleský; Witold Pawlowski; et al. CREATION OF A 3D STRUCTURE BASED ON THE HIGH STRENGTH METALLURGICAL GRAPHENE®. Surface Review and Letters 2019, 26, 1850206, 10.1142/s0218625x18502062.
- Humeyra Mert; Celal Utku Deniz; Cengiz Baykasoglu; Monte Carlo simulations of hydrogen adsorption in fullerene pillared graphene nanocomposites. Molecular Simulation 2020, 46, 650-659, 10.1080/08927022.2020.1758696.
- Celal Utku Deniz; Humeyra Mert; Cengiz Baykasoglu; Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: A computational study. Computational Materials Science 2021, 186, 110023, 10.1016/j.commatsci.2020.110023.
- Yurong Liu; Zongqiang Zhang; Tianyu Wang; Enhanced hydrogen storage performance of three-dimensional hierarchical porous graphene with nickel nanoparticles. International Journal of Hydrogen Energy 2018, 43, 11120-11131, 10.1016/j.ijhydene.2018.04.202.
- Nikolaos Kostoglou; Chi-Wei Liao; Cheng-Yu Wang; Junko N. Kondo; Christos Tampaxis; Theodore Steriotis; Konstantinos Giannakopoulos; Athanassios G. Kontos; Steve Hinder; Mark Baker; et al. Effect of Pt nanoparticle decoration on the H2 storage performance of plasma-derived nanoporous graphene. Carbon 2021, 171, 294-305, 10.1016/j.carbon.2020.08.061.
- US10858755B2
- Harish Banda; Sandy Périé; Barbara Daffos; Pierre-Louis Taberna; Lionel Dubois; Olivier Crosnier; Patrice Simon; Daniel Lee; Gaël De Paëpe; Florence Duclairoir; et al. Sparsely Pillared Graphene Materials for High-Performance Supercapacitors: Improving Ion Transport and Storage Capacity. ACS Nano 2019, 13, 1443-1453, 10.1021/acsnano.8b07102.
- Lukasz Kaczmarek; Piotr Kula; Tomasz Warga; Lukasz Kolodziejczyk; Petr Louda; Karolína Borůvková; Piotr Niedzielski; Witold Szymanski; Lukáš Voleský; Witold Pawlowski; et al. CREATION OF A 3D STRUCTURE BASED ON THE HIGH STRENGTH METALLURGICAL GRAPHENE®. Surface Review and Letters 2019, 26, 1850206, 10.1142/s0218625x18502062.
- Humeyra Mert; Celal Utku Deniz; Cengiz Baykasoglu; Monte Carlo simulations of hydrogen adsorption in fullerene pillared graphene nanocomposites. Molecular Simulation 2020, 46, 650-659, 10.1080/08927022.2020.1758696.
- Celal Utku Deniz; Humeyra Mert; Cengiz Baykasoglu; Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: A computational study. Computational Materials Science 2021, 186, 110023, 10.1016/j.commatsci.2020.110023.
- Yurong Liu; Zongqiang Zhang; Tianyu Wang; Enhanced hydrogen storage performance of three-dimensional hierarchical porous graphene with nickel nanoparticles. International Journal of Hydrogen Energy 2018, 43, 11120-11131, 10.1016/j.ijhydene.2018.04.202.
- Nikolaos Kostoglou; Chi-Wei Liao; Cheng-Yu Wang; Junko N. Kondo; Christos Tampaxis; Theodore Steriotis; Konstantinos Giannakopoulos; Athanassios G. Kontos; Steve Hinder; Mark Baker; et al. Effect of Pt nanoparticle decoration on the H2 storage performance of plasma-derived nanoporous graphene. Carbon 2021, 171, 294-305, 10.1016/j.carbon.2020.08.061.
- US10858755B2
This entry is offline, you can click here to edit this entry!
