Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Dyslipidemia is a major traditional risk factor for cardiovascular disease (CVD) in chronic kidney disease (CKD) patients, although the altered lipid profile does not explain the number and severity of CVD events. High-density lipoprotein (HDL) is a heterogeneous (size, composition, and functionality) population of particles with different atherogenic or atheroprotective properties. Further studies are warranted to clarify if different HDL subpopulations present different atheroprotective effects.
- HDL subpopulations
- cardiovascular disease risk
- chronic kidney disease
1. Introduction
Chronic kidney disease (CKD) is characterized by a decline in kidney function and/or altered renal structure, causing a gradual to permanent loss of kidney function over time. In end-stage renal disease (ESRD), the worst stage of CKD, the loss of kidney function is irreversible, and patients require renal replacement therapy for survival.
CKD has a major effect on global death risk, being an important cause of morbidity and mortality worldwide [1]. In CKD patients, cardiovascular disease (CVD) events are the most frequent cause of death, with ESRD patients presenting the highest risk of mortality due to CVD [2]. According to the Global Burden Disease of Chronic Kidney Disease Collaboration report, kidney disease per se is an important independent risk factor for CVD [1]. Undeniably, there is a close relationship between CKD and CVD—the dysfunction of one organ causes dysfunction of the other, culminating in the failure of both organs, which is known as cardiorenal syndrome.
The high prevalence of traditional and non-traditional CVD risk factors has been pointed to as an explanation for the high incidence of CVD in CKD patients. In fact, the traditional CVD risk factors alone do not explain the high CV risk in CKD [3]. Moreover, standard clinical interventions for CVD management that are successful in the general population are often unsuccessful in reducing the mortality rate in CKD patients [3]. In the more severe stages of CKD, coronary artery disease (CAD), congestive heart failure, arrhythmias, and sudden cardiac death seem to be important causes of CV mortality [4].
Dyslipidemia is one of the major traditional risk factors for CVD in the general population, and the same occurs in CKD patients, showing dyslipidemia due to alterations in lipid metabolism. The most common changes in lipid profile include an increase in triglycerides (TG), lipoprotein (a), and oxidized lipids, and a reduction in high-density lipoprotein cholesterol (HDLc) values. Hypertriglyceridemia may be explained by an increase in apolipoprotein (Apo) C-III and impairment of very-low-density lipoprotein (VLDL) catabolism, due to lipoprotein lipase deficiency/dysfunction [5]. Both decreased production of Apo A-I and reduced activity of lecithin-cholesterol acyltransferase (LCAT) contribute to the reduction in HDL production observed in CKD [6]. In line with these changes, a rise in TG/HDLc values has been pointed to as a predictor of poor CVD outcome in CKD patients [7]. Decreased values of total cholesterol and low-density lipoprotein cholesterol (LDLc) have been also observed in ESRD patients [8]. These alterations in the lipid profile of CKD patients do not completely account for the number and severity of CVD events, which remain inappropriately high. Interventions in ESRD patients on dialysis with statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) provide limited protection for CVD events, despite the reduction in LDLc levels that is usually achieved [9].
HDL, as well as LDL, is a heterogeneous population of particles with different atherogenic or atheroprotective properties. It has been hypothesized that lipoproteins’ qualities (size, composition, and functionality) may be more important as CVD risk factors than their total circulating levels. HDLc and LDLc concentrations per se may not entirely reflect a beneficial or a risk profile for CVD. Data regarding the biology of these lipoproteins indicate that their subpopulations present different atherogenic/atheroprotective properties [10,11,12,13,14].
2. HDL Subpopulations and CVD Risk
Atherogenic alterations in lipoprotein subpopulations, with less functional HDL and LDL subpopulations, have been reported in several conditions associated with CVD risk, such as CAD, acute coronary syndrome (ACS), incident diabetes, and hypertension [10,11,12,13,14].
HDL is the smallest and the densest lipoprotein (density from 1.063 to 1.21 g/mL), composed of, approximately, 55% protein, 3–15% TG, 26–46% phospholipids, 15–30% cholesterol esters, and 2–10% cholesterol [15]. HDL functions comprise reverse cholesterol transport, as well as anti-inflammatory, anti-apoptotic, anti-thrombotic, and antioxidant effects [16]. HDL includes several subpopulations, differentiated by density, size, charge, and protein and lipid composition; this heterogeneous group of HDL subfractions is believed to present different roles in reverse cholesterol transport and different antioxidant and/or anti-inflammatory properties.
A reduction in size and an increase in density of the HDL subpopulations have been associated with less functionality; large HDL subpopulations have been inversely associated with atherosclerosis development, while small HDL subpopulations were positively correlated with risk for CVD. Indeed, it was reported that individuals with increased CVD risk show lower amounts of larger HDL subpopulations and higher amounts of small HDL subpopulations [17,18], suggesting that larger HDL are more functional and atheroprotective. Similarly, studies in patients with CAD showed an enhancement in small HDL subpopulations [19] and a reduction in large HDL subpopulations, which appear to be inversely correlated with very early CAD [12]. Patients with ACS and with stable CAD showed dysfunctional HDL subpopulations, with different HDL composition (especially HDL3 in ACS), and reduced anti-inflammatory potential [11]. In patients with hypertension, a decrease in large HDL subpopulations and an increase in small HDL subfractions, was also reported [14]. The same changes in large HDL subpopulations were reported for patients with incident diabetes [13] and, in small HDL subpopulations for patients with ACS and with diabetes [20]. Recently, it was observed that patients with advanced type 2 diabetes, when compared to newly diagnosed diabetic patients and controls, showed lower levels of intermediate HDL subpopulation, suggesting that HDL subpopulations may change along the disease [21]. Subjects with lower extremity artery disease, without diabetes mellitus and without hypolipidemic therapy, presented significantly higher small HDL subpopulations [22].
A study in individuals with an atherogenic lipoprotein profile revealed that males have significantly higher levels of small HDL subfractions than females [23].
In metabolic syndrome HDL3c, a small and dense HDL, poor in Apo A-I, showed a defective protection of endothelial cells from apoptosis induced by oxidized LDL (oxLDL) [24]. Obese patients presented decreased large HDL and increased small HDL; however, after laparoscopic adjustable gastric banding, beneficial changes occurred, with an enhancement in large HDL and a reduction in small HDL subpopulations [25]. Interventions improving diet and physical activity in obese patients were also associated with a reduction in small HDL subpopulations [26].
An association between HDL subpopulations and TG levels has been reported, suggesting that increasing values of TG favor the development of smaller HDL subfractions [27]; it was hypothesized that the increase in TG interferes with HDL maturation and with reverse cholesterol transport [27]. Another study in hypertriglyceridemic patients, with lower large HDL and higher small HDL levels, suggested that the abnormal distribution of HDL subpopulations was a consequence of the impaired activity of lipoprotein lipase and LCAT that reduces esterification of free cholesterol of HDL, inducing abnormal HDL maturation and eventually compromising reverse cholesterol transport [28].
Concerning the impact of lipid lowering agents on HDL subpopulations, it was reported that statins, niacin and cholesteryl ester transfer protein (CETP) inhibitors, appear to increase the levels of large HDL [29,30,31]. However, no advantages on HDL subpopulation profile with statins treatment were reported [21]; it was also found that pitavastatin treatment did not lead to significant effects on HDL subfractions; however, a decrease in small and intermediate LDL subpopulations was observed [32].
Although several data suggest that large HDL subpopulations present a more protective effect than small HDL, this is not a consensual concept, and doubts about which HDL subpopulation is more atheroprotective remain [33].
It has been suggested that larger HDL subpopulations are less anti-inflammatory in some conditions; in fact, total HDL concentration decreases in the acute phase of septic shock, along with a shift towards large HDL subpopulations, suggesting a dysfunction in these lipoproteins [34]. Small HDL, namely HDL3, were found to inhibit the expression of vascular cell adhesion molecule 1 in human umbilical vein endothelial cells, more successfully than HDL2, the larger and less dense subpopulations [35]. Moreover, smaller and denser HDL (HDL3b and HDL3c) seem to inhibit the oxidation of LDL more efficiently than HDL2 [36]. Thus, the association between HDL subpopulations and CVD risk seems to be complex and deserves further investigation. Of note, we should not exclude that the controversial data on lipoprotein subpopulations may result from the use of different techniques to evaluate HDL and LDL subfractions, as well as from the use of different characterizations and nomenclature of lipoprotein subfractions for similar methods [37].
HDL subpopulations can be separated and identified by several laboratory techniques, such as ultracentrifugation, nuclear magnetic resonance (NMR) spectroscopy, and electrophoresis, among others [15,16]. By density gradient ultracentrifugation, two main subpopulations, HDL2 (the larger and less dense) and HDL3, can be isolated; gradient gel electrophoresis is able to discriminate HDL into HDL2b (the largest), HDL2a, HDL3a, HDL3b, and HDL3c (the smallest) subpopulations [38]; by agarose gel electrophoresis, HDL can be divided into α-migrating subfractions and pre-β migrating subfractions; 2D gel electrophoresis, a combination of agarose gel with native gradient polyacrylamide gel electrophoresis, can separate α- and β-migrating subpopulations into further subfractions (pre-β1, pre- β2, α1, α2, α3, α4, pre- α1, pre- α2, pre- α3) [39]. The HDL subpopulations can also be isolated according to their composition in their main proteins—HDL subpopulations containing Apo A-I and HDL subpopulations containing both Apo A-II and Apo A-I [40].
The Lipoprint® kit from Quantimetrix Corp. (Redondo Beach, CA, USA), approved by the Food and Drug Administration as a diagnostic tool, involves a non-denaturing, linear, polyacrylamide gel electrophoresis, followed by a complete data acquisition and quantification of lipoprotein subpopulations, using the Lipoprint System. Using this method, HDL is separated into 10 subpopulations that are classified as large HDL (1–3 subpopulations), intermediate HDL (4–7 subpopulations), and small HDL (8–10 subpopulations) [8,25]. In spite of the detection of 26 different-sized HDL subfractions by NMR spectroscopy, the HDL profile is usually reported including only the values for large, medium, and small HDL subpopulations [38].
The exact functions of the different HDL subpopulations, as well as their role in different clinical conditions, are, thus, still poorly clarified.
3. HDL Subpopulations in CKD
In a recent study by our group, in ESRD patients on dialysis, we found higher values of large HDL and lower intermediate and small HDL levels (Figure 1; Figure 1b vs. Figure 1a), despite lower (vs. control—Figure 1a) HDLc concentration [8]. Using the same technique (Lipoprint® kit), identical profiles of HDL subpopulations in ESRD patients were reported by others [41,42]. In our study [8], oxLDL and oxLDL/LDLc were inversely correlated with large HDL and positively correlated with intermediate and small HDL subpopulations, in agreement with the concept that HDL subpopulations have different antioxidant activities. We also found that adiponectin correlated positively with large HDL and negatively with intermediate and small HDL, suggesting that the enhancement in adiponectin induces a beneficial change, by favoring an increase in large HDL and a decrease in intermediate and small HDL subpopulations [8]. However, as stated previously, the possibility that the alterations in size and cholesterol composition of HDL subpopulations are not accompanied by improvement in their functionality cannot be ruled out. In normotriglyceridemic patients on hemodialysis (HD), a distribution of HDL towards an increase in large and less dense subpopulations, HDL2, was also reported [43].
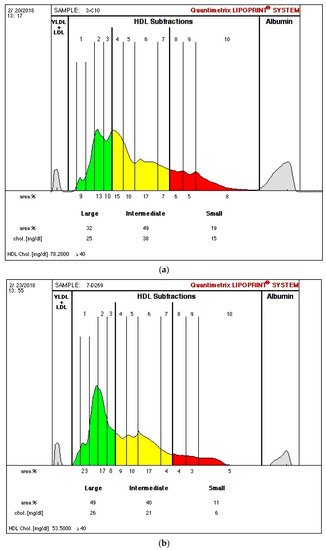
Figure 1. Illustration of high-density lipoprotein (HDL) separation into subfractions of one studied control (a) and one end-stage renal disease patient on dialysis (b) using the Lipoprint® kit from Quantimetrix Corp. (Redondo Beach, CA, USA). (HDL is separated into 10 subfractions that are classified as large HDL (1–3 subfractions—green color), intermediate HDL (4–7 subfractions—yellow color), and small HDL (8–10 subfractions—red color)).
Tsuzaki et al. [44] studied the association of adiponectin levels and of a single nucleotide polymorphism (SNP) of the adiponectin gene with the size of HDL subpopulations. The authors observed that adiponectin levels and SNP276 of the adiponectin gene may condition the size of HDL subpopulations; small HDL was found to correlate negatively, significantly, and independently with adiponectin and the SNP276 G allele [44]. These findings [44] and our data [8] suggest that a close relationship exists between adiponectin and HDL subpopulations.
As referred to previously, HDL can be also divided into HDL containing Apo A-I (but not Apo A-II) and HDL containing both Apo A-I and ApoA-II, which seems to be less cardioprotective [40]. Pre-β1-HDL is a minor subfraction of HDL containing Apo A-I, that contains neither Apo A-II nor the initial acceptor of cellular cholesterol in the process of reverse cholesterol transport [41]. Enhanced levels of pre-β1-HDL were observed in patients with stages 3a, 3b, and 4 of CKD, which clearly reveals disturbances in the metabolism of HDL in kidney disease [45]. Moreover, it was reported that, as the estimated glomerular filtration rate decreases, the ratio pre-β1-HDL/HDL containing Apo A-I without Apo A-II increases [45].
Controversially, Alabakovska et al. reported that in ESRD patients, HDL2b, the larger subpopulation, was reduced and HDL3c was increased [46]. Another study reported that HDL2 was significantly decreased in both CKD and ESRD patients, while HDL3 was higher in ESRD patients, as compared to controls [47].
Kidney transplantation, a common option for the treatment of ESRD patients, has been associated with improvement of the lipid profile [48]; however, data indicate that dyslipidemia still persists after transplantation [49]. Concerning HDL subfractions after kidney transplantation, Stefanovic et al. [50], conducting a study in children and adolescents with ESRD, reported that non-transplanted patients had lower mean HDL particle size, lower percentages of HDL2b, and higher percentages of HDL3a, 3b, and 3c subpopulations than post-transplantation patients, suggesting an atheroprotective effect for kidney transplantation [50].
A shift in HDL size towards larger subpopulations was found in patients with proteinuria [51], suggesting that proteinuria is associated with the loss of small HDL by the kidney; however, the impact of these alterations in cholesterol transport were not evaluated. It was also reported that HDL3 subpopulations decrease and oxidized HDL2 augments with increasing severity of CKD [52].
Doubts concerning which HDL subpopulation(s) are more or less atheroprotective still remain.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050554
This entry is offline, you can click here to edit this entry!
