Obesity is a prevalent disease worldwide, usually associated with infertility. Studies in obese infertile females show the occurrence of systemic hyperestrogenemia, hyperinsulinemia, and associated ovarian dysfunction through premature follicular atresia and anovulation [1]. Indeed, the ovaries of obese mothers have been shown to accumulate lipids, high levels of reactive oxygen species [2], and inflammatory mediators [3]. Furthermore, obesity has been shown to hamper not only oocyte maturation and quality [4] but also embryo development [5], with reported long term effects and direct causality between obesity in the mother and prevalence of cardiovascular disease or cancer in the offspring.
- leptin
- obesity
- ovary
- folliculogenesis
- oocyte
- Introduction
The worldwide epidemic of obesity has reached unprecedented levels and infertility is described as an associated comorbidity [1]. Indeed, obese women were linked to poor reproductive outcomes, such as anovulation or decreased conception rate [6]. Literature has evidenced the link between peripheral insulin resistance and functional hyperandrogenism and hyperestrogenism [7], the main causes of anovulation and reduced endometrial receptivity [1]. Studies in mice have shown how obesity-associated hyperinsulinemia and diabetes can lead to delayed oocyte maturation in preovulatory follicles and apoptosis in granulosa cells (GC) [8]. Furthermore, ovaries of obese women were shown to present high levels of androstenedione and testosterone [7], responsible for premature follicular atresia. Therefore, the endocrine imbalance observed in obese mothers affects directly the follicular pool, reducing the number of primordial follicles and compromising fertility [9].
- Leptin - A Common Denominator between Ovarian Function and Obesity
During obesity progression, the ever-growing adipose tissue secretes large amounts of leptin, which cause systemic hormonal imbalance. Leptin is mainly known to regulate appetite at central level [10], besides modulating gonadotropin releasing hormone (GnRH) neurons activity and gonadotropins release [11]. Nonetheless, leptin is also an important modulator of ovarian function [12]. Leptin long and short receptor isoforms were detected in most cell types in murine ovary, particularly in the oocyte [12]. Likewise, both isoforms of leptin receptor (ObR) were previously detected in human GC and theca cells (TC) [13], as well as leptin and leptin soluble receptor in human follicular fluid [13, 14]. Thus, leptin signaling components’ heavy representation in the ovary makes the organ particularly vulnerable to the systemic hyperleptinemia observed in obese mothers [15].
Concerning the role of leptin in the ovary, evidences gathered from studies with leptin or ObR deficient mice, as well as women, confirmed their infertility and altered pubertal development [16, 17]. Functionally, leptin actions in the ovary were shown to have a bimodal nature. In vivo and in vitro studies in mouse ovarian explants [18] evidenced a dose-dependent effect on progesterone (P4) synthesis, with low doses stimulating and high doses inhibiting expression of enzymes involved in P4 synthesis [18]. Also, studies in other species corroborated aforementioned observations, as the in vitro treatment of equine luteal cells with lower doses of leptin supported P4 secretion, whereas higher doses presented no effect [19]. Particularly regarding follicular dynamics, studies in mice showed that high levels of circulating leptin blocked folliculogenesis, but lower circulating levels of leptin supported the transition from primary to secondary follicle [20]. Conversely, the in vitro treatment of mouse follicles with mouse recombinant leptin once more revealed a dose-dependent response with higher treatment doses inhibiting follicular growth [21]. Presently, we used the data from Zhang and co-workers’ recen3t report profiling the transcriptome GC and oocytes from different stages of folliculogenesis in women [22], and plotted the main components of leptin signaling pathway in order to understand if their expression profile could be linked to leptin concerted role at particular developmental stages. Indeed, we confirmed some of leptin signaling components like protein tyrosine phosphatase non- receptor type 2 (PTPN2), protein tyrosine phosphatase (PTP) 1B, or STAT3 were abundantly transcribed in both oocytes and GC throughout folliculogenesis, whereas others, such as SOCS3, presented very low expression in both cell types under physiological conditions, particularly in later stages of folliculogenesis (Figure 1). Finally, we have recently shown that obesity progression in diet- induced obese (DIO) mice alters leptin signaling in the ovary with increased leptin signaling in the ovary of 4 weeks (wk) DIO mice, being followed by the establishment of leptin resistance in the ovaries of 16 wk DIO mice [23]. Indeed, expression levels of SOSC3 in ovarian extracts were dramatically increased already at 4 wk DIO [23]. Therefore, our findings, as well as the observations on leptin signaling components expression in oocytes and GCs from women’s follicles, clearly suggest that the impairment of leptin signaling in the ovaries of obese mothers may contribute to pathogenesis of ovarian failure, particularly given leptin’s established role in ovarian function.
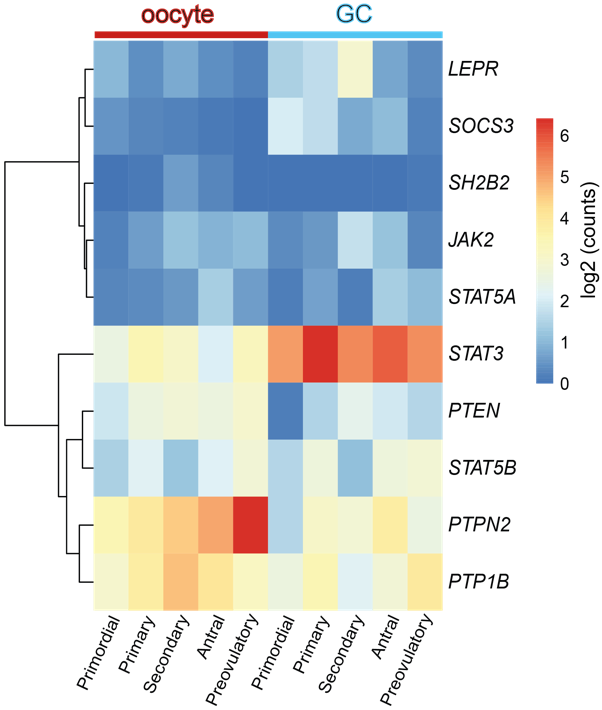
Figure 1. Heatmap representing the expression level of transcripts from leptin signaling pathway components in human oocyte and granulosa cells (GC), throughout folliculogenesis. Primordial = primordial follicle; Primary = primary follicle; Secondary = secondary follicle; Antral = antral follicle; Preovulatory = preovulatory follicle. Color code from blue to red indicates the relative gene expression level from low to high, respectively. Data from Zhang et al 2018 [22]. Leptin receptor (LEPR), suppressor of cytokine signaling 3 (SOCS3), SH2B adaptor protein 1 (SH2B1), Janus kinase 2 (JAK2), signal transducer and activator of transcription (STAT), protein tyrosine phosphatase non- receptor type 2 (PTPN2), protein tyrosine phosphatase (PTP) 1B.
- Ovarian Function in Obese Mothers
The complex nature of folliculogenesis regulation certainly accounts for the vulnerability of the ovaries to the hormonal imbalance observed in obese mothers [6]. Generally, obesity may affect the oocyte and somatic cells at each single developmental stage of folliculogenesis, with the incidence of obesity earlier in life posing a greater threat for the quality of the gamete later in adulthood [24]. Hence, in this section we revisit folliculogenesis, analysing major cellular events taking place throughout the long journey the female gamete makes from primordial follicles until fertilisation, based on lessons learnt from studies in mice and in humans.
3.1 Obesity and Primordial Follicle Activation
Studies in rodents have highlighted the effects of maternal obesity on ovarian failure and, particularly on PI3K dysregulation. A report in rats showed DIO treatment led to premature ovarian failure (POF) through activation of mTOR and suppression of sirtuin (SIRT) 1 signaling [25], whereas the ovaries of mice fed high- fat diet (HFD) presented aberrant gene expression levels of PI3K pathway components [26]. Indeed, the putative crosstalk between leptin and PI3K pathway can be anticipated, mainly through components of insulin signaling cascade. After ObRb activation, JAK2 can phosphorylate IRs, with subsequent activation of PI3K pathway [27]. This generates PIP3, which further activates PIP3-dependent serine/threonine kinases, like PDK1,2, responsible for activation of Akt as previously discussed [27]. Indeed, a study in mice with a triple mutation in ObR tyrosines linked follicle loss to the activation of PTEN/PI3K/Akt/mTOR signaling (Figure 2) [28]. Furthermore, Panwar and colleagues showed the direct effects of leptin on the follicular pool, once passive immunization of prepubertal mice against leptin prompted the transition of primordial to primary follicles [20]. Therefore, adequate levels of leptin signaling in the ovary seem to prevent primordial follicle hyperactivation and help to maintain the follicular pool. Concordantly, these observations suggest the decrease in leptin signaling we have reported in the ovaries of 16 wk DIO mice [23] might accelerate the activation and depletion of primordial follicle pool.
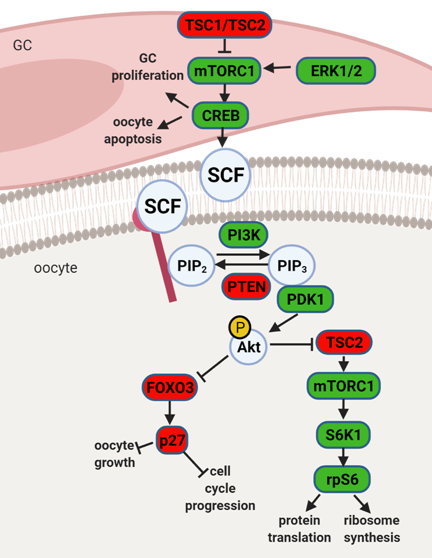
Figure 2. Schematic representation of mammalian target of rapamycin (mTOR) and phosphatidylinositol 3 kinase (PI3K) signaling pathway and its downstream regulators in the granulosa cells (GC) and oocyte. Extracellular signal- regulated protein kinase ½ (ERK1/2) activates mTOR complex 1 (mTORC1) in pre-GC to initiate the activation of primordial follicles. mTORC1 activates cyclic AMP- response element binding protein (CREB), what promotes stem cell factor (SCF) transcription and stimulates PI3K signaling but also affects pre-GC proliferation and oocyte apoptosis. mTOR signaling negative regulators TSC1 and TSC2 suppress mTORC1 activity. PI3K signaling pathway is activated by SCF in the oocyte. PI3K phosphorylates Phosphatidylinositol-4,5-biphosphate (PIP2) to Phosphatidylinositol-3,4,5-triphosphate (PIP3), which interacts with 3- phosphoinositide dependent protein kinase 1 (PDK1) for subsequent phosphorylation of protein kinase B (Akt) and forkhead box O3 (FOXO3) with its downstream mediator cyclin- dependent kinase inhibitor 1B (p27). Upon phosphorylation the inhibitory effect of FOXO3 and p27 on cell cycle progression and oocyte growth is inhibited, and primordial follicle is recruited. Akt also activates ribosomal protein S6 kinase beta-1 (S6K1) through inhibition of TSC2 with subsequent mTORC1 activation and further ribosomal protein S6 (rpS6) phosphorylation, which leads to protein translation and ribosome synthesis. Factors indicated in red are associated with follicle dormancy, molecules indicated in green associated with follicle activation. Created with BioRender.com.
3.2 Obesity and Preovulatory Follicle Formation
Obesity has been strongly associated with increased circulating levels of both estradiol (E2) and androgens, not only due to the ability of the adipose tissue to synthesize steroids [29], but also to low circulating sex hormone binding globulin levels and suppression of gonadotropin release. Moreover, ovarian TC are known to respond to insulin during androgen synthesis [1]. Seminal work by Wu and co-workers clearly showed the link between hyperinsulinemia in DIO mice and increased ovarian androgen production through hyperactivation of CYP17 in TC [30]. On the other hand, Souter et al. also correlated gain in body mass index (BMI) with E2 secretion by preovulatory follicle in obese women [31]. Finally, studies in rats evidenced the link between obesity and the lack of preovulatory surge of P4 and LH [32]. Hence, obesity clearly disrupts steroidogenesis, predisposing to premature follicular atresia and anovulation.
3.2 Oocyte Maturation and Early Embryo Development in Obese Mothers
Obesity leads to severe systemic hormonal imbalance with drastic consequences for oocyte maturation and embryo development. Firstly, maternal obesity alters insulin, glucose and free fatty acids concentration in follicular fluid, directly affecting oocyte metabolism and reducing oocyte maturation [33]. Secondly, insulin- stimulated glucose uptake was shown to be impaired in cumulus cells (CC) isolated from mice treated with HFD, suggesting the establishment of insulin-resistance [34]. Indeed, activation of polyol pathway during hyperglycaemia was shown to negatively affect metabolism and CC- oocyte communication [35]. As a result, in obese mothers oocyte maturation, fertilisation rate and embryo quality were significantly decreased [36]. Moreover, oocytes derived from obese mice after in vitro fertilisation and culture presented reduced development [36]. Furthermore, studies in mice clearly linked maternal obesity with oocyte and zygote increased mitochondrial potential, mitochondrial DNA content and biogenesis and generation of reactive oxygen species (ROS) [2]. Importantly, the absence of mitophagy was presented as the main cause of mitochondrial dysfunction in oocytes and early embryos from obese mothers [37]. Another recent report also linked lack of expression of Stella in oocytes from obese mice with increased hydroxymethylation in the zygote and DNA instability [5]. Thus, obesity drastically affects both oocyte and embryo quality.
4 Conclusion
Folliculogenesis regulation is a complex process that depends on the crosstalk between local and systemic factors, accounting, therefore, for its vulnerability to maternal physiological fitness. Indeed, leptin, an established local regulator of folliculogenesis, presents increased circulating levels in obese mothers, with major consequences for follicular activation, recruitment and growth. In addition to increased ovarian ObRb activation and altered leptin signaling, deleterious effects of systemic hyperleptinemia during obesity can also result from local overexpression of mediators of leptin resistance, such as SOCS3. As a result, leptin’s prominent role as suppressor of follicular pool activation can be affected in obese mothers, with consequent POF. During preovulatory follicle formation, altered leptin signaling affects not only steroidogenesis but the communication between GC and oocyte, which is known to be key for antrum formation and oocyte growth. Finally, changes in leptin signaling and impaired metabolism may also incur drastic consequences for oocyte maturation, hampering meiosis resumption and cytoplasmic maturation (Figure 3). Hence, advances in our understanding on the role of leptin on ovarian pathophysiology during obesity should unravel innovative tools to monitor the quality of the oocyte during disease progression, potentially preventing pregnancy failure and ensuring the birth of a healthy offspring.
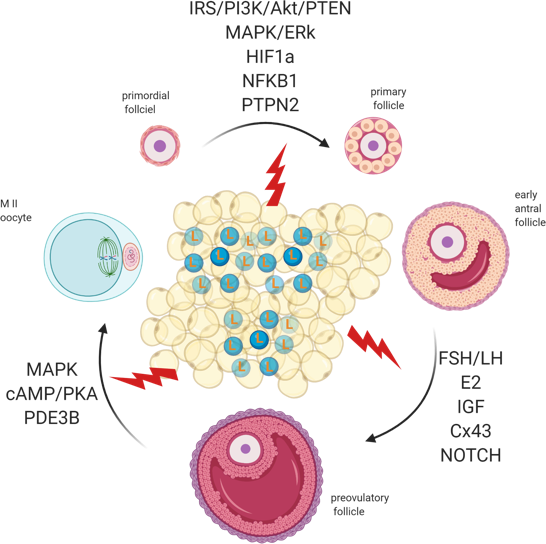
Figure 3. Adipose tissue secretes excessive amounts of leptin (L) during obesity. Leptin signaling in the ovaries of obese mothers is altered, culminating with the establishment of leptin resistance. As a result, signalling pathways governing primordial follicle activation, preovulatory follicle formation and oocyte maturation can be affected. Created with BioRender.com.
REFERENCES
[1] A. Gambineri, D. Laudisio, C. Marocco, S. Radellini, A. Colao, and S. Savastano, “Female infertility: which role for obesity?,” Int. J. Obes. Suppl., vol. 9, no. 1, pp. 65–72, Apr. 2019.
[2] N. Igosheva et al., “Maternal Diet-Induced Obesity Alters Mitochondrial Activity and Redox Status in Mouse Oocytes and Zygotes,” PLoS One, vol. 5, no. 4, p. e10074, Apr. 2010.
[3] R. L. Robker, L. L.-Y. Wu, and X. Yang, “Inflammatory pathways linking obesity and ovarian dysfunction,” J. Reprod. Immunol., vol. 88, no. 2, pp. 142–148, Mar. 2011.
[4] X. Yang et al., “Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes,” Fertil. Steril., vol. 97, no. 6, pp. 1438–1443, Jun. 2012.
[5] L. Han et al., “Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes,” Nat. Genet., vol. 50, no. 3, pp. 432–442, Mar. 2018.
[6] Z. Ö. Dağ and B. Dilbaz, “Impact of obesity on infertility in women.,” J. Turkish Ger. Gynecol. Assoc., vol. 16, no. 2, pp. 111–7, 2015.
[7] M. Cree-Green et al., “Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids,” Am. J. Physiol. Metab., vol. 308, no. 9, pp. E726–E733, May 2015.
[8] A. S. Chang, A. N. Dale, and K. H. Moley, “Maternal Diabetes Adversely Affects Preovulatory Oocyte Maturation, Development, and Granulosa Cell Apoptosis,” Endocrinology, vol. 146, no. 5, pp. 2445–2453, May 2005.
[9] M. E. Skaznik-Wikiel, D. C. Swindle, A. A. Allshouse, A. J. Polotsky, and J. L. McManaman, “High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice1,” Biol. Reprod., vol. 94, no. 5, May 2016.
[10] C. Vaisse, J. L. Halaas, C. M. Horvath, J. E. Darnell, M. Stoffel, and J. M. Friedman, “Leptin activation of Stat3 in the hypothalamus of wild–type and ob/ob mice but not db/db mice,” Nat. Genet., vol. 14, no. 1, pp. 95–97, Sep. 1996.
[11] A. K. Odle et al., “Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence,” Front. Endocrinol. (Lausanne)., vol. 8, no. JAN, p. 367, Jan. 2018.
[12] N. K. Ryan, C. M. Woodhouse, K. H. Van der Hoek, R. B. Gilchrist, D. T. Armstrong, and R. J. Norman, “Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation.,” Biol. Reprod., vol. 66, no. 5, pp. 1548–54, May 2002.
[13] C. Karlsson et al., “Expression of Functional Leptin Receptors in the Human Ovary 1,” J. Clin. Endocrinol. Metab., vol. 82, no. 12, pp. 4144–4148, Dec. 1997.
[14] C. K. Welt, A. L. Schneyer, K. Heist, and C. S. Mantzoros, “Leptin and Soluble Leptin Receptor in Follicular Fluid,” J. Assist. Reprod. Genet., vol. 20, no. 12, pp. 495–501, Dec. 2003.
[15] M. Maffei et al., “Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects,” Nat. Med., vol. 1, no. 11, pp. 1155–1161, 1995.
[16] K. Clément et al., “A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction,” Nature, vol. 392, no. 6674, pp. 398–401, Mar. 1998.
[17] X. Tu et al., “The Influence of LepR Tyrosine Site Mutations on Mouse Ovary Development and Related Gene Expression Changes,” PLoS One, vol. 10, no. 11, p. e0141800, Nov. 2015.
[18] M. G. Bilbao, M. P. Di Yorio, and A. G. Faletti, “Different levels of leptin regulate different target enzymes involved in progesterone synthesis,” Fertil. Steril., vol. 99, no. 5, pp. 1460–1466, Apr. 2013.
[19] A. Galvão et al., “Opposing roles of leptin and Ghrelin in the equine corpus luteum regulation: An in vitro study,” Mediators Inflamm., vol. 2014, 2014.
[20] S. Panwar, M. Herrid, K. G. Kauter, and J. R. McFarlane, “Effect of passive immunization against leptin on ovarian follicular development in prepubertal mice,” J. Reprod. Immunol., vol. 96, no. 1–2, pp. 19–24, Dec. 2012.
[21] J. E. Swain, R. L. Dunn, D. McConnell, J. Gonzalez-Martinez, and G. D. Smith, “Direct Effects of Leptin on Mouse Reproductive Function: Regulation of Follicular, Oocyte, and Embryo Development,” Biol. Reprod., vol. 71, no. 5, pp. 1446–1452, Nov. 2004.
[22] Y. Zhang et al., “Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions,” Mol. Cell, vol. 72, no. 6, pp. 1021-1034.e4, Dec. 2018.
[23] K. Wołodko, E. Walewska, M. Adamowski, J. Castillo-Fernandez, G. Kelsey, and A. Galvão, “Leptin resistance in the ovary of obese mice is associated with profound changes in the transcriptome of cumulus cells,” Cell. Physiol. Biochem., vol. 54, no. 3, pp. 417–437, 2020.
[24] Y. He et al., “Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: The childhood determinants of adult health study and the Bogalusa heart study,” Hum. Reprod., vol. 35, no. 5, pp. 1185–1198, May 2020.
[25] N. Wang et al., “Obesity accelerates ovarian follicle development and follicle loss in rats,” Metabolism., vol. 63, no. 1, pp. 94–103, Jan. 2014.
[26] J. Nteeba, J. W. Ross, J. W. Perfield, and A. F. Keating, “High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression,” Reprod. Toxicol., vol. 42, pp. 68–77, Dec. 2013.
[27] K. Hegyi, “Leptin-induced signal transduction pathways,” Cell Biol. Int., vol. 28, no. 3, pp. 159–169, Mar. 2004.
[28] H. Xia, R. Zhang, H. Guan, and W. Zhang, “Follicle loss and PTEN/PI3K/mTOR signaling pathway activated in LepR-mutated mice,” Gynecol. Endocrinol., vol. 35, no. 1, pp. 44–48, Jan. 2019.
[29] M. Quinkler, B. Sinha, J. W. Tomlinson, I. J. Bujalska, P. M. Stewart, and W. Arlt, “Androgen generation in adipose tissue in women with simple obesity - A site-specific role for 17β-hydroxysteroid dehydrogenase type 5,” J. Endocrinol., vol. 183, no. 2, pp. 331–342, Nov. 2004.
[30] S. Wu et al., “Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell,” Diabetes, vol. 63, no. 4, pp. 1270–1282, Apr. 2014.
[31] I. Souter, L. M. Baltagi, D. Kuleta, J. D. Meeker, and J. C. Petrozza, “Women, weight, and fertility: The effect of body mass index on the outcome of superovulation/intrauterine insemination cycles,” Fertil. Steril., vol. 95, no. 3, pp. 1042–1047, Mar. 2011.
[32] S. C. Sagae et al., “Early onset of obesity induces reproductive deficits in female rats,” Physiol. Behav., vol. 105, no. 5, pp. 1104–1111, Mar. 2012.
[33] S. H. Purcell and K. H. Moley, “The impact of obesity on egg quality,” in Journal of Assisted Reproduction and Genetics, 2011, vol. 28, no. 6, pp. 517–524.
[34] S. H. Purcell, M. M. Chi, and K. H. Moley, “Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex,” Endocrinology, vol. 153, no. 5, pp. 2444–2454, May 2012.
[35] M. L. Sutton-McDowall, R. B. Gilchrist, and J. G. Thompson, “The pivotal role of glucose metabolism in determining oocyte developmental competence,” Reproduction, vol. 139, no. 4. BioScientifica, pp. 685–695, 01-Apr-2010.
[36] R. L. Robker, “Evidence that obesity alters the quality of oocytes and embryos,” Pathophysiology, vol. 15, no. 2. Elsevier, pp. 115–121, 01-Aug-2008.
[37] A. L. Boudoures et al., “Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy,” Dev. Biol., vol. 426, no. 1, pp. 126–138, Jun. 2017.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22084270
