Coenzyme Q10 (CoQ10) has a number of important roles in the cell that are required for optimal functioning of the immune system. These include its essential role as an electron carrier in the mitochondrial respiratory chain, enabling the process of oxidative phosphorylation to occur with the concomitant production of ATP, together with its role as a potential lipid-soluble antioxidant, protecting the cell against free radical-induced oxidation. Furthermore, CoQ10 has also been reported to have an anti-inflammatory role via its ability to repress inflammatory gene expression. Recently, CoQ10 has also been reported to play an important function within the lysosome, an organelle central to the immune response.
- coenzyme Q10
- mitochondria
- lysosome
- inflammation
- reactive oxygen species
- oxidative stress
1. Introduction
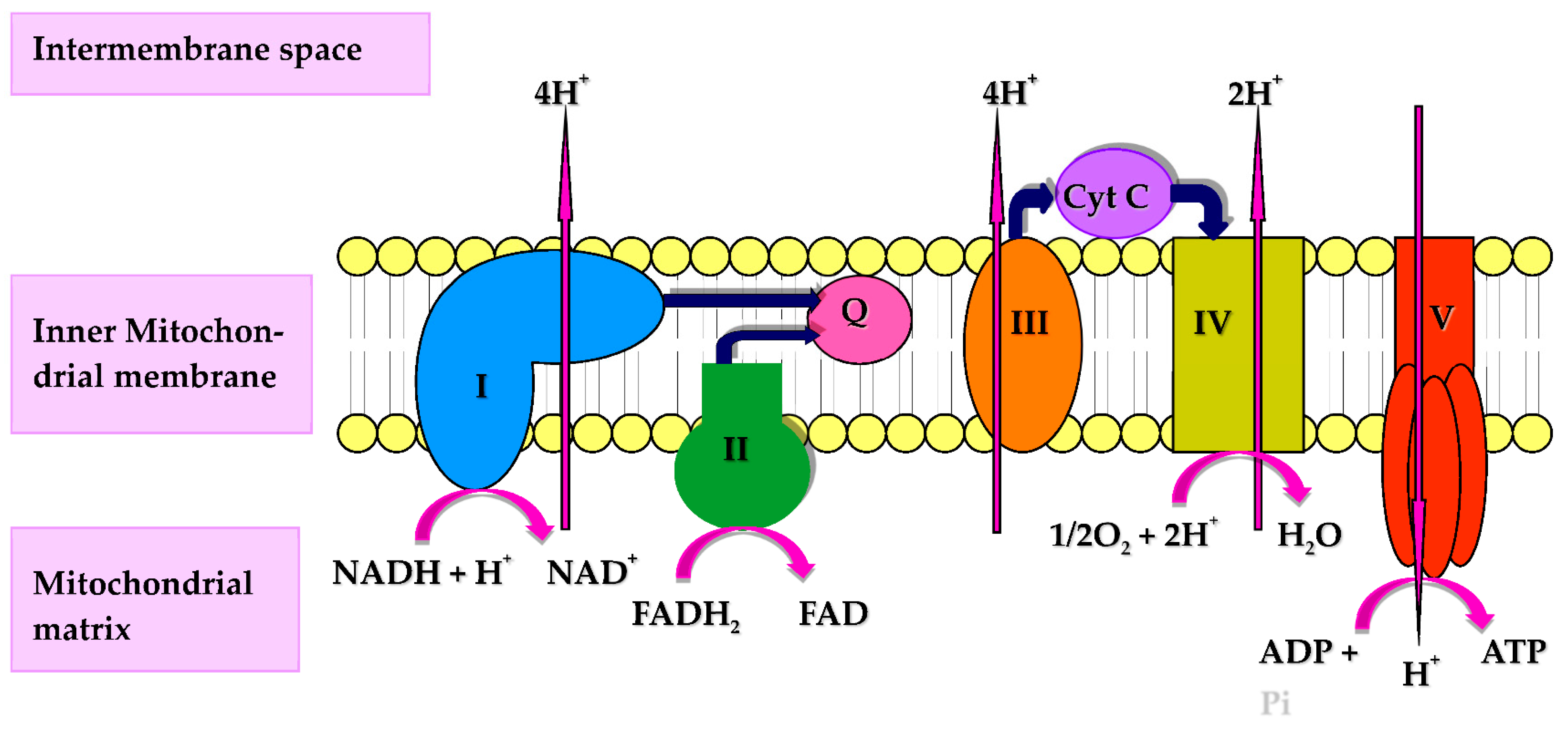 Figure 1. Diagram of the mitochondrial respiratory chain (MRC) and complex V illustrating proton (H+) movement during oxidative phosphorylation. Q: Coenzyme Q10; Cyt C: Cytochrome c.
Figure 1. Diagram of the mitochondrial respiratory chain (MRC) and complex V illustrating proton (H+) movement during oxidative phosphorylation. Q: Coenzyme Q10; Cyt C: Cytochrome c.2. Coenzyme Q10 and Immune Function
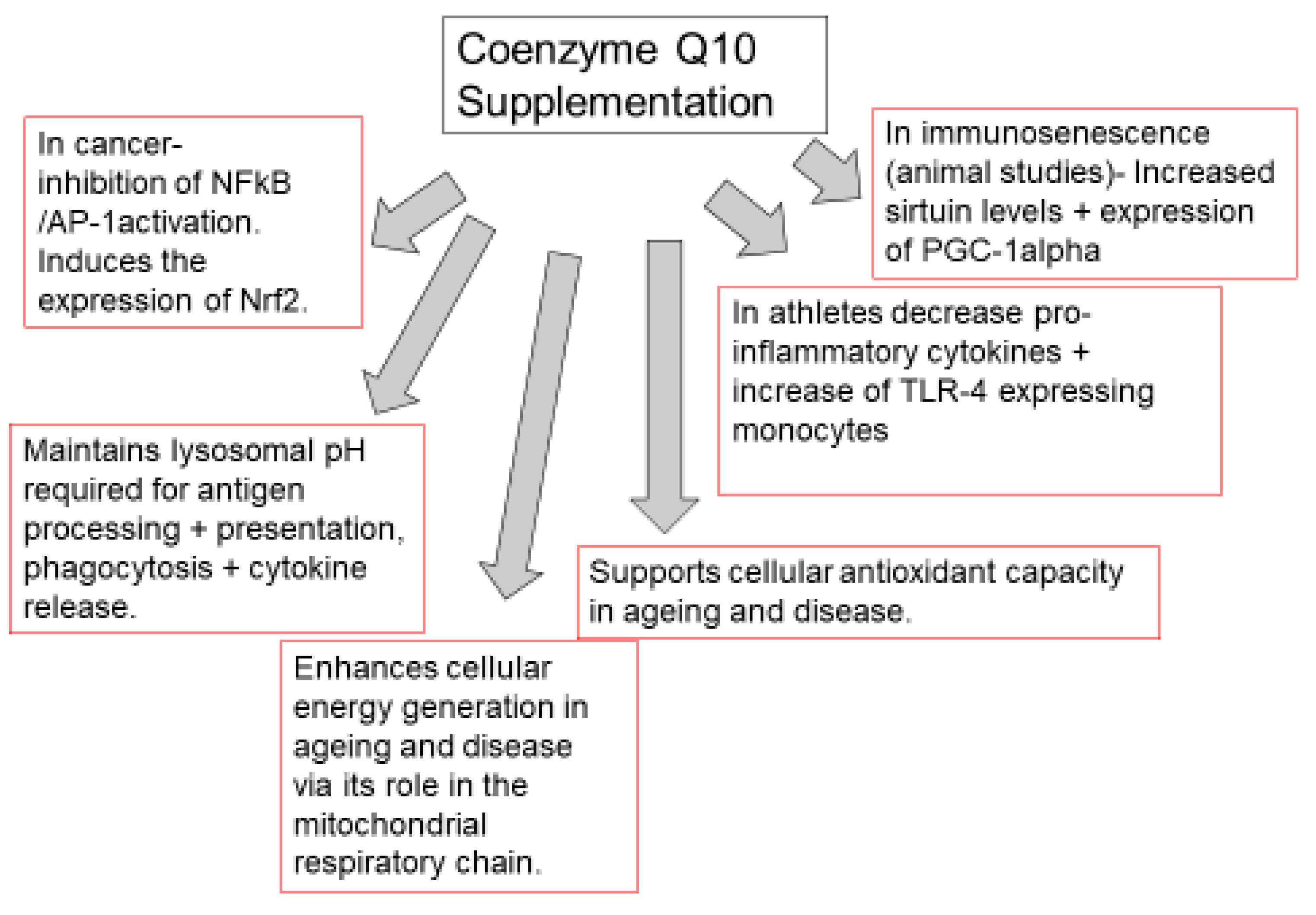 Figure 2. Functions of coenzyme Q10 supplementation in immunopathy, ageing, cancer, and immune function in athletes. TLR-4: toll-like receptor 4 (TLR-4). NFkB: nuclear factor kappa B; AP-1: Activator protein-1. PGC-1 alpha: peroxisome proliferator-activated receptor coactivator 1 alpha. Nuclear factor erythroid 2-related factor 2.
Figure 2. Functions of coenzyme Q10 supplementation in immunopathy, ageing, cancer, and immune function in athletes. TLR-4: toll-like receptor 4 (TLR-4). NFkB: nuclear factor kappa B; AP-1: Activator protein-1. PGC-1 alpha: peroxisome proliferator-activated receptor coactivator 1 alpha. Nuclear factor erythroid 2-related factor 2.3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/antiox10050759
References
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23.
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. et Biophys. Acta (bba)-Mol. Cell Biol. Lipids 2015, 1851, 397–413.
- Hargreaves, I.P. Ubiquinone: Cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003, 40, 207–218.
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598.
- Rosen, G.M.; Pou, S.; Ramos, C.L.; Cohen, M.S.; Britigan, B.E. Free radicals and phagocytic cells. FASEB J. 1995, 9, 200–209.
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183.
- Farough, S.; Karaa, A.; Walker, M.; Slate, N.; Dasu, T.; Verbsky, J.; Fusunyan, R.; Canapari, C.; Kinane, T.; Van Cleave, J. Coenzyme Q10 and immunity: A case report and new implications for treatment of recurrent infections in metabolic diseases. Clin. Immunol. 2014, 155, 209–212.
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.-Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid. Redox Signal. 2014, 20, 2606–2620.
- Fan, L.; Feng, Y.; Chen, G.-C.; Qin, L.-Q.; Fu, C.-l.; Chen, L.-H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136.
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176.
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236.
- Israel, A.; Schäffer, A.A.; Cicurel, A.; Feldhamer, I.; Tal, A.; Cheng, K.; Sinha, S.; Schiff, E.; Lavie, G.; Ruppin, E. Large population study identifies drugs associated with reduced COVID-19 severity. MedRxiv 2020.
- Gvozdjakova, A.; Klauco, F.; Kucharska, J.; Sumbalova, Z. Is mitochondrial bioenergetics and coenzyme Q10 the target of a virus causing COVID-19? Bratisl. Lek. Listy 2020, 121, 775–778.
- Heaton, R.A.; Heales, S.; Rahman, K.; Sexton, D.W.; Hargreaves, I. The Effect of Cellular Coenzyme Q(10) Deficiency on Lysosomal Acidification. J. Clin. Med. 2020, 9, 1923.
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866.
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Investigators, Q.-S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649.
- Mantle, D. Coenzyme Q10 supplementation for diabetes and its complications: An overview. Br. J. Diabetes 2017, 17, 145–148.
- Mantle, D.; Hargreaves, I.P. Coenzyme Q10 supplementation in non-alcoholic fatty liver disease: An overview. J. Prescr. Pract. 2020, 2, 200–204.
- Hargreaves, I.; Mantle, D.; Milford, D. Chronic kidney disease and coenzyme Q10 supplementation. J. Kidney Care 2019, 4, 82–90.
- Mantle, D.; Hargreaves, I.P. Ataxia and coenzyme Q10: An overview. Br. J. Neurosci. Nurs. 2018, 14, 108–114.
