Breast cancer is a leading cause of cancer-related deaths worldwide, predominantly caused by metastasis. It is generally accepted that the pattern of breast cancer metastasis is largely determined by the interaction between the chemokine receptors on cancer cells and the chemokines expressed at the sites of metastatic disease. Chemokine receptors belong to the G-protein-coupled receptors (GPCRs) family that appear to be implicated in inflammatory diseases, tumor growth and metastasis. One of its members, C-C Chemokine receptor 7 (CCR7), binds chemokines CCL19 and CCL21, which are important for tissue homeostasis, immune surveillance and tumorigenesis. These receptors have been shown to induce the pathobiology of breast cancer due to their ability to induce cellular proliferation and migration upon the binding of the cognate chemokine receptors. The underlying signaling pathways and exact cellular interactions within this biological system are not fully understood and need further insights.
- CCR7
- CCL19
- CCL21
- breast cancer
- metastasis
- signaling therapy
1. Introduction
2. Chemokine Receptors in Cancer
3. C-C Chemokine Receptor 7 (CCR7) and Breast Cancer
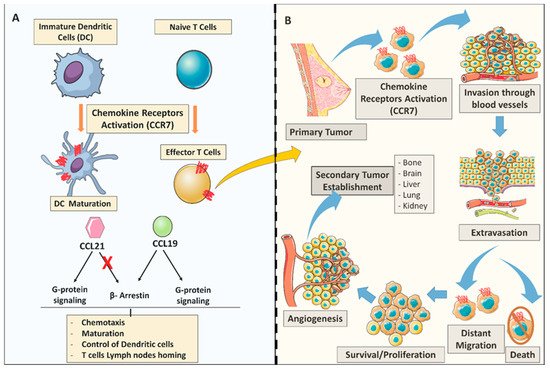
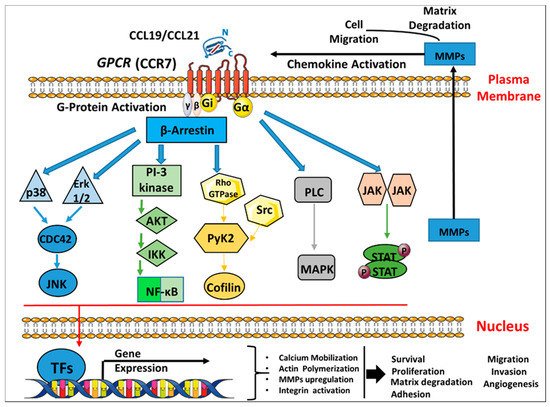
3.1. Molecular Aspects of C-C Chemokine Receptor 7 (CCR7) Signaling Cascades in Breast Cancer
3.2. The Expression and Functional Role of C-C Chemokine Receptor 7 (CCR7) in Breast Cancer Cells in Vitro
3.3. The Role of C-C Chemokine Receptor 7 (CCR7) on Breast Cancer Metastasis in Vivo
This entry is adapted from the peer-reviewed paper 10.3390/cancers12041036
References
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568.
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242.
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. An atypical addition to the chemokine receptor nomenclature: IUPHAR Review 15. Br. J. Pharmacol. 2015, 172, 3945–3949.
- Sánchez-Madrid, F.; Del Pozo, M.A. Leukocyte polarization in cell migration and immune interactions. EMBO 1999, 18, 501–511.
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. BBA 2014, 1843, 2563–2582.
- Sozzani, S.; Allavena, P.; D’Amico, G.; Luini, W.; Bianchi, G.; Kataura, M.; Imai, T.; Yoshie, O.; Bonecchi, R.; Mantovani, A. Cutting edge: Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. Immunology 1998, 161, 1083–1086.
- Forster, R.; Emrich, T.; Kremmer, E.; Lipp, M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 1994, 84, 830–840.
- Heydtmann, M.; Adams, D. Understanding selective trafficking of lymphocyte subsets. Gut 2002, 50, 150–152.
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82.
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606.
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702.
- Blanchet, X.; Langer, M.; Weber, C.; Koenen, R.R.; von Hundelshausen, P. Touch of chemokines. Front. Immunol. 2012, 3, 175.
- Stone, M.J.; Hayward, J.A.; Huang, C.E.; Huma, Z.; Sanchez, J. Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Med. 2017, 18, 342.
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559.
- Tanaka, T.; Bai, Z.; Srinoulprasert, Y.; Yang, B.; Hayasaka, H.; Miyasaka, M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005, 96, 317–322.
- Zhou, J.; Xiang, Y.; Yoshimura, T.; Chen, K.; Gong, W.; Huang, J.; Zhou, Y.; Yao, X.; Bian, X.; Wang, J.M. The role of chemoattractant receptors in shaping the tumor microenvironment. BioMed Res. Int. 2014, 2014, 751392.
- Sarvaiya, P.J.; Guo, D.; Ulasov, I.; Gabikian, P.; Lesniak, M.S. Chemokines in tumor progression and metastasis. Oncotarget 2013, 4, 2171.
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56.
- Morris, V.L.; MacDonald, I.C.; Koop, S.; Schmidt, E.E.; Chambers, A.F.; Groom, A.C. Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: Videomicroscopic analysis. Clin. Exp. Metastasis 1993, 11, 377–390.
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144.
- Kruizinga, R.C.; Bestebroer, J.; Berghuis, P.; de Haas, C.J.; Links, T.P.; de Vries, E.G.; Walenkamp, A.M. Role of chemokines and their receptors in cancer. Curr. Pharm. Des. 2009, 15, 3396–3416.
- Luker, K.E.; Luker, G.D. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006, 238, 30–41.
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin. Cancer Res. 2005, 11, 5686–5693.
- Kochetkova, M.; Kumar, S.; McColl, S. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009, 16, 664–673.
- Wilson, J.L.; Burchell, J.; Grimshaw, M.J. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006, 66, 11802–11807.
- Mori, T.; Kim, J.; Yamano, T.; Takeuchi, H.; Huang, S.; Umetani, N.; Koyanagi, K.; Hoon, D.S. Epigenetic up-regulation of CC chemokine receptor 7 and CXC chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005, 65, 1800–1807.
- Wu, S.; Lu, X.; Zhang, Z.; Lei, P.; Hu, P.; Wang, M.; Huang, B.; Xing, W.; Jiang, X.; Liu, H. CC chemokine ligand 21 enhances the immunogenicity of the breast cancer cell line MCF-7 upon assistance of TLR2. Carcinogenesis 2011, 32, 296–304.
- Chen, Y.; Stamatoyannopoulos, G.; Song, C.-Z. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res. 2003, 63, 4801–4804.
- Hattermann, K.; Gebhardt, H.; Krossa, S.; Ludwig, A.; Lucius, R.; Held-Feindt, J.; Mentlein, R. Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling. Elife 2016, 5, e10820.
- Lacalle, R.A.; Blanco, R.; Carmona-Rodriguez, L.; Martin-Leal, A.; Mira, E.; Manes, S. Chemokine receptor signaling and the hallmarks of cancer. Int. Rev. Cell Mol. Biol. 2017, 331, 181–244.
- Wang, J.; Knaut, H. Chemokine signaling in development and disease. Development 2014, 141, 4199–4205.
- Curnock, A.P.; Logan, M.K.; Ward, S.G. Chemokine signalling: Pivoting around multiple phosphoinositide 3-kinases. Immunology 2002, 105, 125–136.
- Bonecchi, R.; Mollica Poeta, V.; Capucetti, A.; Massara, M. Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front. Immunol. 2019, 10, 379.
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007, 11, 526–538.
- Kehrl, J.H. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol. Res. 2006, 34, 211–227.
- Legler, D.F.; Thelen, M. New insights in chemokine signaling. F1000 Res. 2018, 7.
- Kobayashi, D.; Endo, M.; Ochi, H.; Hojo, H.; Miyasaka, M.; Hayasaka, H. Regulation of CCR7-dependent cell migration through CCR7 homodimer formation. Sci. Rep. 2017, 7, 1–14.
- Lu, Y.; Wang, H.; Mills, G.B. Targeting PI3K-AKT pathway for cancer therapy. Rev Clin Exp Hematol. 2003, 7, 205–228.
- Sotsios, Y.; Ward, S.G. Phosphoinositide 3-kinase: A key biochemical signal for cell migration in response to chemokines. Immunol. Rev. 2000, 177, 217–235.
- Rolin, J.; Maghazachi, A.A. Implications of chemokine receptors and inflammatory lipids in cancer. ImmunoTargets Ther. 2014, 3, 9.
- Wong, M.; Fish, E.N. RANTES and MIP-1α activate stats in T cells. J. Biol. Chem. 1998, 273, 309–314.
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.-F.; Chen, Z.; Zu, X.-B. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE 2015, 10, e0119506.
- Miyagaki, T.; Sugaya, M.; Murakami, T.; Asano, Y.; Tada, Y.; Kadono, T.; Okochi, H.; Tamaki, K.; Sato, S. CCL11–CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 2011, 71, 2056–2065.
- Brust, T.F.; Conley, J.M.; Watts, V.J. Gαi/o-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur. J. Pharmacol. 2015, 763, 223–232.
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639.
- Watts, A.O.; Verkaar, F.; van der Lee, M.M.; Timmerman, C.A.; Kuijer, M.; van Offenbeek, J.; Van Lith, L.H.; Smit, M.J.; Leurs, R.; Zaman, G.J. β-Arrestin recruitment and G protein signaling by the atypical human chemokine decoy receptor CCX-CKR. J. Biol. Chem. 2013, 288, 7169–7181.
- López-Giral, S.; Quintana, N.E.; Cabrerizo, M.; Alfonso-Pérez, M.; Sala-Valdés, M.; de Soria, V.G.G.; Fernández-Rañada, J.M.; Fernández-Ruiz, E.; Muñoz, C. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J. Leukoc. Biol. 2004, 76, 462–471.
- Cunningham, H.D.; Shannon, L.A.; Calloway, P.A.; Fassold, B.C.; Dunwiddie, I.; Vielhauer, G.; Zhang, M.; Vines, C.M. Expression of the CC chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl. Oncol. 2010, 3, 354–361.
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Li, X.; Huang, X.-W.; Wang, Z.; Fan, J.; Dai, Z.; Zhou, J. CXCR2/CXCL5 axis contributes to epithelial–mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 2015, 358, 124–135.
- Ma, H.; Gao, L.; Li, S.; Qin, J.; Chen, L.; Liu, X.; Xu, P.; Wang, F.; Xiao, H.; Zhou, S. CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 2015, 6, 24348.
- Raju, R.; Gadakh, S.; Gopal, P.; George, B.; Advani, J.; Soman, S.; Prasad, T.K.; Girijadevi, R. Differential ligand-signaling network of CCL19/CCL21-CCR7 system. Database 2015, 2015, bav106.
- Sonbul, S.N.; Gorringe, K.L.; Aleskandarany, M.A.; Mukherjee, A.; Green, A.R.; Ellis, I.O.; Rakha, E.A. Chemokine (C-C motif) receptor 7 (CCR7) associates with the tumour immune microenvironment but not progression in invasive breast carcinoma. J. Pathol. 2017, 3, 105–114.
- Wu, J.; Li, L.; Liu, J.; Wang, Y.; Wang, Z.; Wang, Y.; Liu, W.; Zhou, Z.; Chen, C.; Liu, R. CC chemokine receptor 7 promotes triple-negative breast cancer growth and metastasis. ABBS 2018, 50, 835–842.
- Xu, B.; Zhou, M.; Qiu, W.; Ye, J.; Feng, Q. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial–mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med. 2017, 6, 1062–1071.
- Al Akoum, C.; Akl, I.; Rouas, R.; Fayyad-Kazan, M.; Falha, L.; Renno, T.; Burny, A.; Lewalle, P.; Fayyad-Kazan, H.; Badran, B. NFAT-1, Sp-1, Sp-3, and miR-21: New regulators of chemokine C receptor 7 expression in mature human dendritic cells. Hum. Immunol. 2015, 76, 307–317.
- Mburu, Y.K.; Egloff, A.M.; Walker, W.H.; Wang, L.; Seethala, R.R.; Van Waes, C.; Ferris, R.L. Chemokine receptor 7 (CCR7) gene expression is regulated by NF-κB and activator protein 1 (AP1) in metastatic squamous cell carcinoma of head and neck (SCCHN). J. Biol. Chem. 2012, 287, 3581–3590.
- Li, C.; Wang, Z.; Chen, Y.; Zhou, M.; Zhang, H.; Chen, R.; Shi, F.; Wang, C.; Rui, Z. Transcriptional silencing of ETS-1 abrogates epithelial-mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol. Rep. 2015, 33, 559–565.
- Fang, L.-W.; Kao, Y.-H.; Chuang, Y.-T.; Huang, H.-L.; Tai, T.-S. Ets-1 enhances tumor migration through regulation of CCR7 expression. BMB Rep. 2019, 52, 548.
- Vahedi, L.; Ghasemi, M.; Yazdani, J.; Ranjbar, S.; Nouri, B.; Alizadeh, A.; Afshar, P. Investigation of CCR7 Marker Expression Using Immunohistochemical Method and Its Association with Clinicopathologic Properties in Patients with Breast Cancer. Hematology 2018, 12, 103.
- Zabicki, K.; Colbert, J.A.; Dominguez, F.J.; Gadd, M.A.; Hughes, K.S.; Jones, J.L.; Specht, M.C.; Michaelson, J.S.; Smith, B.L. Breast cancer diagnosis in women≤ 40 versus 50 to 60 years: Increasing size and stage disparity compared with older women over time. Ann. Surg. Oncol. 2006, 13, 1072–1077.
- Li, X.; Sun, S.; Li, N.; Gao, J.; Yu, J.; Zhao, J.; Li, M.; Zhao, Z. High expression of CCR7 predicts lymph node metastasis and good prognosis in triple negative breast cancer. Cell. Physiol. Biochem. 2017, 43, 531–539.
- Gracio, F.; Burford, B.; Gazinska, P.; Mera, A.; Noor, A.M.; Marra, P.; Gillett, C.; Grigoriadis, A.; Pinder, S.; Tutt, A. Splicing imbalances in basal-like breast cancer underpin perturbation of cell surface and oncogenic pathways and are associated with patients’ survival. Sci. Rep. 2017, 7, 1–14.
- Chen, X.; Wu, J.; Huang, H.; Ding, Q.; Liu, X.; Chen, L.; Zha, X.; Liang, M.; He, J.; Zhu, Q. Comparative profiling of triple-negative breast carcinomas tissue glycoproteome by sequential purification of glycoproteins and stable isotope labeling. Cell. Physiol. Biochem. 2016, 38, 110–121.
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550.
- Kodama, J.; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann. Oncol. 2007, 18, 70–76.
- Pan, M.-R.; Hou, M.-F.; Chang, H.-C.; Hung, W.-C. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008, 283, 11155–11163.
- Tamamura, H.; Hori, A.; Kanzaki, N.; Hiramatsu, K.; Mizumoto, M.; Nakashima, H.; Yamamoto, N.; Otaka, A.; Fujii, N. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003, 550, 79–83.
- Andre, F.; Cabioglu, N.; Assi, H.; Sabourin, J.; Delaloge, S.; Sahin, A.; Broglio, K.; Spano, J.; Combadiere, C.; Bucana, C. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann. Oncol. 2006, 17, 945–951.
- Liu, Y.; Ji, R.; Li, J.; Gu, Q.; Zhao, X.; Sun, T.; Wang, J.; Li, J.; Du, Q.; Sun, B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J. Exp. Clin. Cancer Res. 2010, 29, 16.
- Leung, H.-W.; Zhao, S.-M.; Yue, G.G.-L.; Lee, J.K.-M.; Fung, K.-P.; Leung, P.-C.; Tan, N.-H.; Bik-San Lau, C. RA-XII inhibits tumour growth and metastasis in breast tumour-bearing mice via reducing cell adhesion and invasion and promoting matrix degradation. Sci. Rep. 2015, 5, 16985.
- Pearson, H.B.; Pouliot, N. Modeling metastasis in vivo. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013.
- Bähr, A.; Wolf, E. Domestic animal models for biomedical research. Reprod. Domest. Anim. 2012, 47, 59–71.
- Salazar, N.; Muñoz, D.; Hoy, J.; Lokeshwar, B.L. Use of shRNA for stable suppression of chemokine receptor expression and function in human cancer cell lines. Methods Mol. Biol. 2014, 1172, 209–218.
- Li, F.; Zou, Z.; Suo, N.; Zhang, Z.; Wan, F.; Zhong, G.; Qu, Y.; Ntaka, K.S.; Tian, H. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial–mesenchymal transition in human breast carcinoma. Med. Oncol. 2014, 31, 180.
- Saur, D.; Seidler, B.; Schneider, G.; Algül, H.; Beck, R.; Senekowitsch–Schmidtke, R.; Schwaiger, M.; Schmid, R.M. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005, 129, 1237–1250.
- Takekoshi, T.; Fang, L.; Paragh, G.; Hwang, S.T. CCR7-expressing B16 melanoma cells downregulate interferon-γ-mediated inflammation and increase lymphangiogenesis in the tumor microenvironment. Oncogenesis 2012, 1, e9.
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.-t.; Hwang, S.T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643.
- Sun, L.; Zhang, Q.; Li, Y.; Tang, N.; Qiu, X. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 15729.
- Koizumi, K.; Kozawa, Y.; Ohashi, Y.; Nakamura, E.S.; Aozuka, Y.; Sakurai, H.; Ichiki, K.; Doki, Y.; Misaki, T.; Saiki, I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol. Rep. 2007, 17, 1511–1516.
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16, 1–11.
- Strien, L.; Joensuu, K.; HEIKKILÄ, P.; Leidenius, M.H. Different expression patterns of CXCR4, CCR7, maspin and FOXP3 in luminal breast cancers and their sentinel node metastases. Anticancer Res. 2017, 37, 175–182.
- Weitzenfeld, P.; Kossover, O.; Körner, C.; Meshel, T.; Wiemann, S.; Seliktar, D.; Legler, D.F.; Ben-Baruch, A. Chemokine axes in breast cancer: Factors of the tumor microenvironment reshape the CCR7-driven metastatic spread of luminal-A breast tumors. J. Leukoc. Biol. 2016, 99, 1009–1025.
- Power, C.A. Knock out models to dissect chemokine receptor function in vivo. J. Immunol. Methods 2003, 273, 73–82.
- Houshmand, P.; Zlotnik, A. Therapeutic applications in the chemokine superfamily. Curr. Opin. Chem. Biol 2003, 7, 457–460.
