Alzheimer’s disease (AD) is a neurodegenerative disease associated with human aging. Ten percent of individuals over 65 years have AD and its prevalence continues to rise with increasing age.
- Alzheimer’s disease
- neuroinflammation
- immunosenescence
- inflammasome
- mitochondria
- microglia
- astrocytes
- DAMPs
- SASP
1. Introduction
Aging is characterized by dysregulated immune [1] and metabolic homeostasis [2,3] where there is chronic sterile low-grade inflammation or inflammaging [4] that involves cellular senescence [5,6], immunosenescence [7,8,9,10], mitochondrial dysfunction [11,12], defective autophagy [13,14] and mitophagy [15,16], dysregulation of the ubiquitin–proteasome system [17,18], activation of the DNA damage response [19,20], meta-inflammation or metaflammation from chronic overnutrition or obesity [21,22], and gut microbiota dysbiosis [5,23,24,25]. These are reflected by changes in circulating immune markers including C-reactive protein (CRP) [26], interleukin-6 (IL-6) [27], tumor necrosis factor alpha (TNF-α) [28] and its soluble receptors (tumor necrosis factor receptor I (TNFR-I) and tumor necrosis factor receptor II (TNFR-II)) [28], vascular cell adhesion molecule I (VCAM-I) [29], d-dimer [30], and sirtuin signaling [31,32]. The drawback of chronic subclinical inflammation is that it is an essential risk factor for increasing the incidence of degenerative diseases such as AD [33,34,35]. There are currently an estimated 728 million persons aged 65 years or over in the world. In the next 30 years, this number is expected to more than double to exceed 1.5 billion in 2050 (https://www.un.org/development/desa/pd/news/world-population-ageing-2020-highlights (accessed on 20 March 2021)). Thus, the aging population vulnerable to inflammaging will significantly increase over the next few decades.
AD is a chronic devastating neurodegenerative disorder in which increasing age is the strongest non-modifiable disease risk factor [36,37]. There are currently no effective therapies for AD [38,39]. It is clinically characterized by the progressive deterioration of memory and other cognitive functions [40]. It is the leading cause of dementia, affecting 50 million people worldwide [41]. Its neuropathological hallmarks include extracellular β-amyloid (Aβ) plaques and intracellular hyper-phosphorylated tau (p-τ) in neurofibrillary tangles, accompanied with synaptic and neuronal loss [40,41,42]. AD can be classified as (i) familial or early-onset AD (EOAD) or (ii) sporadic or late-onset AD (LOAD) [43]. Both share almost similar pathophysiology [44,45]. Three causative genes, including presenilin 1 (PSEN1) [46], presenilin 2 (PSEN2) [47], and amyloid precursor protein (APP) [48], are involved in the pathogenesis of EOAD in an autosomal-dominant trait [49,50]. LOAD, however, comprises most AD cases (>95%) where the greatest risk factor is advanced age [51], while the common genetic risk factor is an allelic variation in apolipoprotein E (Apo E) [52]. Recent large scale studies of AD genetics, employing genome-wide association studies (GWAS), whole exome sequencing (WES), and whole genome sequencing (WGS), have defined additional genes whose variants contribute to increased risk [53,54]. These include Clusterin (CLU), Sortilin-related receptor-1 (SORL1), ATP-binding cassette subfamily A member 7 (ABCA7), Bridging integrator 1 (BIN1), phosphatidylinositol binding clathrin assembly protein (PICALM), CD2 associated protein (CD2AP), Complement component (3b/4b) receptor 1 (CR1), CD33, triggering receptor expressed on myeloid cells 2 (TREM2), and phospholipase D3 (PLD3) [55,56,57]. Intriguingly, more than 50% of validated gene variants are implicated in innate immune and microglial functions [58,59,60], including the top two AD risk genes, APOE and TREM2 [61,62]. Epigenomic analysis shows that AD GWAS loci are preferentially enriched in enhancer sequences involved in innate immune processes [63,64] as well as endocytosis, cholesterol/sterol metabolism, and synaptic function [55,65,66]. TREM2 enhances the rate of phagocytosis in microglia and macrophages; modulates inflammatory signaling; and controls myeloid cell number, proliferation, and survival [67], and it has been revealed that triggering TREM2 receptor in microglial cells is closely associated with the pathogenesis of AD [68]. TREM2 modulates microglial functions in response to Aβ plaques and tau tangles [69,70]. In early AD, the absence of TREM2 leads to increased amyloid pathology that progressively becomes worse owing to the loss of phagocytic Aβ clearance [69]. In AD, TREM2 variants arise in part because of their reduced capacity to phagocytose Aβ [71].
While compelling evidence indicates that AD has a multifactorial etiology [72,73,74], neuroinflammation plays a central role in its etiopathogenesis [75,76], owing to its capacity to exacerbate Aβ and τ pathologies [77]. In vivo positron emission tomography (PET) studies provide direct evidence of increased microglia activation (inflammation) in the brains of AD patients [78,79,80]. The levels of pro-inflammatory cytokines in AD patient serum and post mortem brain are elevated [81,82], and Aβ can activate the brains’ innate immune cells [83,84]. The sustained inflammatory response in AD brains [85,86,87] extends beyond a reaction to neuronal loss [88] and involves microglia, astrocytes, oligodendrocytes, mast cells, cytokines, and chemokines, as well as complement [89]. These collectively play an integral role in the onset and progression of the disease [88,90,91]. Other early-onset processes involved in the etiology of the disease include mitochondrial dysfunction resulting in altered glucose metabolism [92] and oxidative stress [93]; chronic hypoperfusion [94]; and neuronal cell cycle re-entry that leads to neuronal tetraploidization (NT) [94], trisomy 21 mosaicism [95], and synapse loss [96]. These processes may synergistically interact to facilitate the neurodegenerative process in AD [93,97].
2. Mediators of Neuroinflammation
2.1. Microglia
Microglia are the CNS’s immune cells [98] and are different from peripheral and other tissue-resident macrophages [99,100,101,102,103,104]. They arise from yolk-sac fetal macrophages and are unique in their capacity for self-renewal [105,106,107]. Other tissue macrophages develop from precursors that emerge later in life [103,104]. They constantly survey their milieu and assess ongoing synaptic activity, mediate synaptic pruning, clear debris, and provide trophic support for neurons [108]. In pathological situations such as chronic stress, the blood brain barrier (BBB) may become compromised, allowing peripheral hematopoietic cells to cross into neural tissue and become part of the parenchymal microglia/macrophage pool [109,110]. In response to CNS insults such as neuronal injury or infection, microglia become activated to produce pro-inflammatory factors (M1 phenotype) or anti-inflammatory factors (M2 phenotype) [111]. An exquisite balance of anti-inflammatory mediators to heal and repair tissues and pro-inflammatory mediators to clear cellular debris and aggregated misfolded proteins is essential for the maintenance of a healthy CNS [27,111]. With advancing age, microglia acquire an activated phenotype and release pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 [112,113,114]. In AD, microglia react to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) [115,116,117] to assume a M1 phenotype, leading to an exacerbation of inflammation and an acceleration of disease progression [88] (Figure 1). Preliminary findings implicate a link between Aβ and neuroinflammation [118]. In AD brain slices, activated microglia surround both extracellular Aβ plaques and neurons containing neurofibrillary tangles (NFTs) [119,120]. It is thought that Aβ activate microglia, which then secrete IL1β, IL6, and TNFα, as well as (C-C motif) ligand (CCL 2/4/11), which lead to the recruitment of more microglia and astrocytes to the Aβ locus [121]. Microglia phagocytize Aβ through a range of cell surface receptors, including cluster of differentiation (CD)-14, toll-like receptor (TLR)-2, TLR4, α6β1 integrin, CD47, and scavenger receptors such as CD36 [122,123,124,125]. In AD, the accumulation of Aβ throughout the brain results partly from the failure of microglia to remove extracellular Aβ [126,127,128] and AD cortical specimens reveal that the microglia surrounding plaques have impaired Aβ uptake [127,129,130]. It has been shown in human and animal studies that inflammation influences APP processing overall [131,132]. Initially, microglial activation may serve to eliminate Aβ [126,133,134,135], but their chronic activation may amplify the amyloid cascade [133,136] and lead to neurotoxicity [137,138]. In rat intraventricular hemorrhage (IVH) model of AD, Aβ accumulation tracks with neuroinflammation and may contribute to the cognitive impairment [139].There is no consensus, however, regarding the relationship between in vivo microglial activation and Aβ plaque burden [140,141,142,143,144]. Aβ has recently been suggested to be an antimicrobial peptide that fibrilizes in order to activate the innate immune defense system and protect the host from a wide range of infectious agents [145].
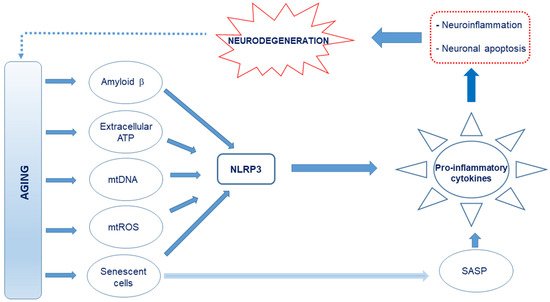
Figure 1. Schematic representation of the proposed causes of neuroinflammation in Alzheimer’s disease (AD). Age-related release of damage-associated molecular patterns (DAMPs) such as Aβ, extracellular ATP, and cell debris such as circulating mitochondrial DNA, which are capable of interacting with the Nod-like receptor 3 (NLRP3), creates an oxidative and neuroinflammatory environment through the excessive production and release of pro-inflammatory cytokines and reactive oxygen and nitrogen species (RONS). Further, mitochondrial reactive oxygen species (mtROS) and senescence-associated secretory phenotype (SASP) factors from the senescent cells, which also drive senescence in nearby cells, produce pro-inflammatory cytokines. This culminates in neuroinflammation and neuronal apoptosis.
2.2. Astrocytes
Astrocytes are the most abundant cell type in the CNS and a critical part of the tripartite synapse [146]. They are highly sensitive to their environment and rapidly respond to CNS needs and insults [147]. They also regulate the maturation of neurons and help maintain their function [147,148]. They are found in various states of activation and can be neuroprotective (reducing inflammation and stimulating repair) or neurotoxic (promoting inflammation that may result in neurodegeneration) [149,150]. They respond to inflammatory molecules such as cytokines and chemokines and are able to detect aggregated proteins such as Aβ [148,151,152]. They hypertrophy upon activation and upregulate glial fibrillary acidic protein (GFAP) expression [153,154]. Reactive astrocytes are a distinct trait of AD patient brains [155] and are also a feature of AD mouse model brains [156,157,158]. Intralaminar astrocytes are atrophied and severely disrupted in post-mortem AD brains [159]. In the 3xTg, which contain three mutations associated with a familial AD (APP Swedish, MAPT P301L, and PSEN1 M146V) mouse model, atrophic astrocytes appear in the entorhinal cortex (EC) as early as 30 days and are present until Aβ plaques begin to emerge at 12 months of age [160]. This phenomenon also occurs in other AD mouse models including the 5xTG-AD, PDAPP-J20, and Swiss 3 mice [161,162,163,164]. When astrocytes are created from familial and sporadic AD-induced pluripotent stem cells (iPSC), they have an atrophic phenotype in vitro [165]. Inhibiting astrogliosis in AD mouse brains results in Aβ accumulation with increased histopathology [166] and they are associated with cognition [167]. This may result in a breach of the blood brain barrier (BBB), leading to an infiltration of peripheral immune cells, aggravating neuroinflammation and inducing neurotoxicity by impairing glutamate homeostasis [168,169], and generating altered Ca2+ signaling [170].
2.3. Oligodendrocytes
The main function of oligodendrocytes is to provide support and insulation to the axons by forming myelin sheaths around nerve fibers. Their involvement in AD is not fully understood, although emerging evidence implicates their potential role in pathogenesis and progression of AD [171]. Tsai et al. recently reported that oligodendrocytes are severely impaired in AD [172] and, indeed, there is focal loss of oligodendrocytes and a reduction in myelin proteins near Aβ plaques [173,174,175]. Aβ not only impairs the survival and maturation of oligodendrocyte progenitor cells (OPCs), but also hampers the formation of the myelin sheath [176]. Neuroinflammation and oxidative stress may also contribute to oligodendrocyte dysfunction and death [175].
2.4. Myeloid Cells Other Than Microglia
The other monocytic cells found in the CNS include perivascular macrophages that line blood vessels of the brain, macrophages within the choroid plexus, and meningeal macrophages in the leptomeninges [177]. Dendritic cells, monocytes, and granulocytes are found in the meninges and are recruited to the brain during or after an insult or other pathology [178,179]. The CNS-resident macrophages express scavenger receptors (SR) and TLRs that facilitate phagocytosis and degradation of Aβ [180,181]. In SR knock out mouse models of AD, Aβ accumulates in the parenchyma and the animals have cognitive deficits [124,182]. In the CD11c-DNR mouse model of AD, which expresses a dominant-negative form of the TGF-β receptor under the control of the CD11c promoter, the brain levels of Aβ are reduced by up to 90%, the Aβ plaques and cerebral vasculature are surrounded by macrophages [183], and the behavior of the animals is significantly improved [183]. The migration of peripheral monocytes is dependent on C-C chemokine receptor type 2 (CCR2) [184]. Blocking transforming growth factor (TGF)-β signaling increases peripheral myeloid cell infiltration into the CNS and significantly reduces Aβ burden [183]. It is still not exactly clear how myeloid infiltration into the brain contributes to damage or clearance of pathological proteins.
3. Defective Autophagy and Neuroinflammation
Cells degrade protein aggregates and damaged organelles by autophagy and defective mitochondria by mitophagy [185,186,187,188]. With advancing age, autophagy gradually subsides and this decline is linked to defective mitochondria and results in inflammaging [189]. Damaged cellular and organelle components that accumulate as a result of inadequate autophagy are released as damage-associated molecular patterns (DAMPs) [190,191,192]. Dysfunctional mitochondria that are not eliminated by mitophagy release large amounts of mitochondrial DNA (mtDNA) into the cytosol and, together with ROS [193,194], metabolites such as ATP, fatty acids, Aβ, succinate, per-oxidized lipids, advanced glycation end-products, altered N-glycans, and HMGB1 are also recognized as DAMPs and trigger an innate immune inflammatory response [195,196] by directly activating TLR9. This initiates the transcription of pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β, and MMP-8 [197] and activates the Nod-like receptor 3 (NLRP3) inflammasome, a key regulator of inflammation [198,199,200], to activate caspase-1 and facilitate IL-1β and IL-18 maturation as well as gasdermin D-mediated pyroptotic cell death [201,202,203]. These inflammatory responses can be blocked by Pro-IL-1β degradation in autophagosomes [204]. Further, the mitochondrial derived peptide (MDP) known as mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) [205] reduces inflammation by inhibiting cytokines such as TNF-α and IL-6, while simultaneously promoting an anti-inflammatory response. MOTS-c stimulates IL-10 as well as signal transducer and activators of transcription 3 (STAT3) and aryl hydrocarbon receptor (Ahr), which inhibit NFκB expression and proinflammatory cytokine production [206]. Another mitochondrial peptide, humanin, also has anti-inflammatory effects [207,208]. The chronic sterile low-grade inflammation elicited [4] may culminate in immunosenescence [209] and compromise neuronal function [32]. This may partly explain why dysregulated NLRP3 inflammasome activation is observed in AD [210,211]). Eliminating damaged and dysfunctional mitochondria by mitophagy may prevent the hyperinflammation triggered by NLRP3 inflammasome activation [212].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050524
