Sample preparation is an essential and preliminary procedure of most chemical analyses. Due to the sample diversity, the selection of appropriate adsorbents for the effective preparation and separation of different samples turned out to be important for the methods. By exploiting the rapid development of material science, some novel adsorption materials, especially graphene-based nanomaterials, have shown supremacy in sample pretreatment. In this review, a discussion between these nanomaterials will be made, as well as some basic information about their synthesis. The focus will be on the different environmental applications that use these materials.
- graphene
- nanomaterials
- sample preparation
- environmental analysis
- magnetic solid phase extraction
- solid phase extraction
- magnetic nanoparticles
1. Introduction
Oftentimes, the concentration of the target compounds may be lower than the limits of detection (LOD) even for the most advanced instruments, especially for trace or ultra-trace analytes in complex matrices. With sample preparation, we eliminate the above problem by preconcentrating the low concentrations of the molecules, thus enhancing the sensitivity and rendering it possible to detect them [1]. While this step is time-consuming and sometimes costly, there are green approaches which lower the cost and the time needed to pretreat a sample. Some extra benefits that sample preparation provides are the removal of contaminants or substances that interfere with the compounds of interest (cleanup), the dissolution of a solid sample into an aqueous media or the chemical modification with the proper reagents so as to obtain better analytical parameters (lower LODs, higher recoveries, higher selectivity and sensitivity, improved accuracy and repeatability and etc.) [2].
Lately, there has been a huge interest in graphene (G) and graphene-based nanomaterials, with scientists all over the world studying and publishing a variety of topics regarding its properties and its use in the sample pretreatment of a plethora of samples, matrices and target analytes [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36]. Some of these applications include wastewater treatment [37], such as carbon dioxide adsorption [38], photocatalysis [39] graphene oxide membranes [40] and so forth. Graphene is a single layer of two dimensions of sp2 hybridized carbon atoms arranged into a honeycomb grid. Due to this formation, G shows extraordinary properties, such as high mechanical strength, excellent optical, great electronic and thermal properties with a vast surface area of 2630 m2/g [35][36]. The combination of its high surface area and its hexagonal array with delocalized π-π electron interactions renders it ideal for the adsorption of aromatic compounds and their derivatives, which also intrigues the analytical chemists to use it as a sorbent [41]. The most common way of producing G is via the chemical oxidation and sonication of graphite to graphene oxide (GO) with the Hummers method and its subsequent reduction of GO to reduced G (rGO) (Figure 1) [42][43]. Organic analytes can be adsorbed onto G with the development of potent π-π interactions between the carbon rings of the analytes and the hexagonal carbons of G [44]. GO provides a variety of functional groups, making electrostatic interactions possible and enabling hydrogen bonding possible for the organic molecules that contain N- and/or O- functional groups. Other possible interactions between the analytes and the nanoparticles are dispersion forces, dative bonds and hydrophobicity [45]. G and GO are oftentimes modified so as to be more selective towards the compounds of interest or to enhance their adsorption properties and recovery rates (Figure 2).
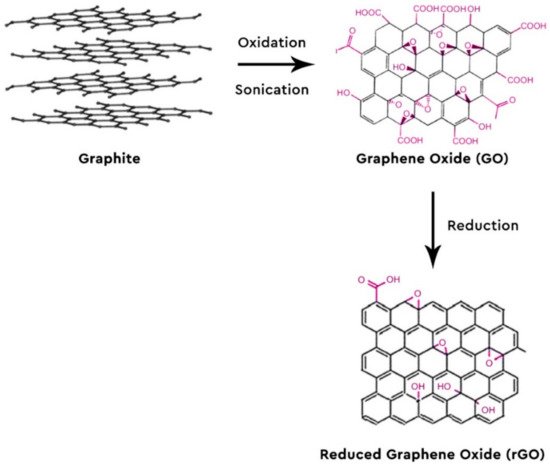
Figure 1. Synthesis of graphene oxide (GO) and reduced G (rGO) from graphite powder.
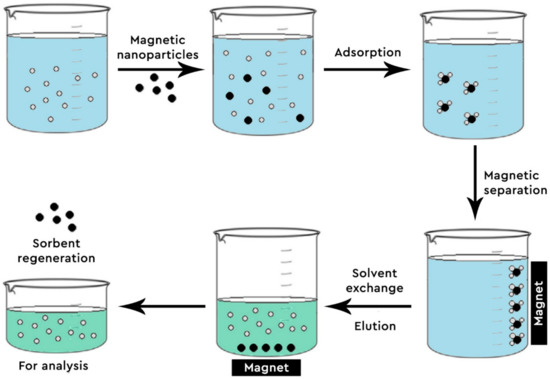
Figure 2. Steps of Magnetic Solid Phase Extraction (MSPE).
2. Graphene-Based Nanomaterials and Nanocomposites
One of the assets provided by graphene-based materials is selective adsorption. The rGO presents a large π-electron system, making it ideal for the adsorption of organic compounds that consist of aromatic rings. The GO has several functional groups that favor the adsorption of organic molecules containing oxygen- or nitrogen-functional groups.
Even though graphene-based materials (G, GO and rGO) have an enhanced ability of absorbing many and different compounds, they show a significant drawback; the difficulty of the material with the adsorbed analytes being separated from the mother solution. To combat this problem, magnetic nanoparticles (MNPs) are impregnated into rGO or GO. Thus, the combination of high adsorption capacity from G and the convenient separation of the adsorbent from the sample, given from the MNPs, makes these nanocomposites ideal for analytical purposes [43]. Moreover, Fe3O4 nanoparticles are non-toxic, have a small particle size, offer high reactivity and possess a vast specific surface area, adding to the benefits of their usage and rendering them more appealing [46][47].
2.1. Synthesis of Magnetic Nanoparticles
The common methods for synthesizing MNPs are co-precipitation, thermal decomposition, hydrothermal synthesis, colloidal and high-energy ball mill. Depending on which method was used, a specific size and dispersion of size distribution of MNPs are obtained [48].
The modified co-precipitation method introduced by Massart includes the addition of FeCl3·6H2O and FeCl2·4H2O in deionized water, with the temperature rising to 60 °C in order to acquire a yellow and clear solution and stirred vigorously. Afterwards, aqueous ammonia is inserted drop by drop until the pH reaches 10. Then, it is left for the reaction to take place for about 30 min, under continuous and vigorous stirring. Throughout the whole experiment, N2 is used as a protective gas. When the reaction finishes, the black precipitate is collected with the help of a strong magnet, it is washed many times with deionized water and ethanol. Lastly, the magnetite nanoparticles are lyophilized [49][50].
The thermal decomposition method carried out by Kluchova et al. produced maghemite nanoparticles by the solid-state isothermal decomposition in air at 400 °C of Fe(C2H3O2)2·2H2O for 1 h. Before the synthesis, the solid was homogenized in an agate mortar, following in the precursor particles having a size distribution of 1–5 μm [51].
Zheng et al. [52] produced Fe3O4 nanoparticles by hydrothermal synthesis, where 1 mmol of Fe(NO3)3·9H2O and 0.5 mmol of sodium bis(2-ethylhexyl)sulfosuccinate were added in 10 mL deionized water, following the addition of 2 mL of 50% hydrazine hydrate while stirring. This solution was put into a 20 mL Teflon-lined stainless-steel autoclave of and heated to 160 °C at a pace of 5 °C/min. For 10 h, the temperature was kept at 160 °C and then the mixture brought to room temperature naturally. The nanoparticles were filtered, washed several times with ethanol and deionized water and air-dried. The morphology and the structure of the MNPs were characterized by TEM, HRTEM and XRD.
Another procedure for synthesizing Fe3O4 magnetic nanoparticles is by the microwave-solvothermal method suggested by Li et al. [53], where 5 mmol of FeCl3·6H2O, 400 mg of Na3C6H5O7 and 50 mmol of NH4CH3CO2 were added in a solution of 70 mL of ethylene glycol. The black solution was stirred and heated and then transferred into a microwave transparent PTFE-TFM-lined alumina ceramic vessel, with the capacity of 100 mL. Then, it was heated for 15 min to 260 °C for 2 h in a microwave reaction system. Finally, the black powder of Fe3O4 was gathered with the help of a strong magnet and washed several times with ethanol.
2.2. Synthesis of Fe3O4-GO Nanocomposites
Kyzas et al. [50] presented two ways of preparing magnetic GO (mGO): by co-precipitation (mGOp) or by impregnation (mGOi). In the former method, graphite oxide is scattered in deionized water with the help of a sonicator for 30 min. Then, specific amounts of FeCl3·6H2O and FeCl2·4H2O are dissolved in deionized water at room temperature and the mixture is added drop by drop to the GO solution, under vigorous stirring and under a N2 atmosphere. After the completion of ion exchange, 28% v/v ammonia solution is added dropwise so the pH reaches the value of 10 for the synthesis of MNPs. Next, the solution is heated to 80 °C and after agitating for 45 min, the black solid is collected via centrifugation, is washed many times with CH3OH and is lyophilized. In the latter method, graphite oxide is again added in deionized water and put into a sonicator for 30 min to obtain GO and then an amount of Fe3O4 nanoparticles are added to the mixture. After another 30 min, a homogenous suspension is obtained and the nanocomposites are collected by centrifugation and freeze-dried.
The aforementioned material was studied for dye adsorption experiments, with Reactive Black 5 (C26H21N5Na4O19S6) being the target compound. From the X-ray diffraction measurements (XRD) presented in the paper, the average crystallite size of the MNPs were found to be around 18.4 nm. Scanning electron microscopy images were taken to identify the morphology of the mGOi nanoparticles and the iron distribution map of the material. Also, the magnetization of the two materials were studied and the mGOi adsorbent had 65 emu/g, which was a bit higher than the same value of mGOp. Lastly, from Fourier transform infrared (FT-IR)spectra, the identification of functional groups, such as carboxylic groups, epoxy groups and ammonium groups, was carried out.
2.3. Synthesis of GO Membranes Composites
Deng et al. [54] firstly prepared hydroxylated graphene (GOH), by using ferrous chloride and hydrogen peroxide at certain volume ratios, while adjusting the pH with diluted HCl, and GO with a modified Hummers method. Then, 1 mg of GO and 1 mg of GOH were mixed into a beaker, under ultrasonication for 30 min to form a uniform dispersion and the solution was put onto the polyethersulfone membranes of pore sizes 0.22 μm and 1 μm under vacuum.
The morphology and validation of the deposition of the material on the membrane was done via scanning electron microscopy (SEM) and X-ray diffraction (XRD). The performance of the adsorption properties was evaluated with dye compounds, such as rhodamine B and methyl orange, and with the protein albumin from bovine serum.
2.4. Synthesis of Graphene Aerogels
Zhi et al. [55] showcased in a review seven different ways of preparing graphene aerogels, either with no templates or with a template. The methods that require no template are the hydrothermal method, where a GO colloidal dispersion is prepared in ethanol or water, heated at a certain temperature and freeze-dried, dried in atmospheric and in supercritical conditions, the chemical reduction method, by using a reducing agent, the chemical cross-linking method, to enhance the interaction between the graphene oxide sheets with the usage of glutaraldehyde or poly(vinyl alcohol), and a 3D printing method, with the preparation of a high viscosity ink of SiO2 and GO suspension. The latter methods thar require a template are the chemical vapor deposition method, where 3D graphene is prepared by the chemical vapor deposition of carbon onto a metal foam, like copper or nickel, the ice template method, by freezing a GO suspension and its gradual melting to form a porous aerogel with GO, and the bubble template method, with the utilization of a surfactant under vigorous stirring so that the GO is trapped in the bubbles and begins to form.
2.5. Synthesis of GO Alginate Beads
Arshad et al. [56] prepared alginate beads of GO by adding 0.2 g sodium alginate in a 5 wt.% GO suspension in deionized water and then deposited dropwise into a 5 wt.% calcium chloride solution. The alginate beads that were formed were kept in the solution for 3 h. These beads were used for the removal of metals and organic compounds, with maximum adsorption capacities reaching 588.2 mg/g bead.
3. Analytical Applications in Environmental Samples
The environmental applications of graphene-based nanomaterials and nanocomposites are classified into two categories: in the extraction and determination of (i) organic pollutants and (ii) metal ions and rare earth elements. Some of the organic compounds that are examined with these materials are Polycyclic Aromatic Hydrocarbons (PAHs), estrogens, sulfonamides, chlorophenols and so forth, whilst the second category consists of Hg, Cr(III), Cd, Cr(VI), Au, Co, Cu, Zn, Pb(II), Ce(III), Tl(III) and other [57][58]. (Table 1, Table 2)
Table 1. Recent applications of graphene-based nanomaterials in environmental analysis.
|
Adsorbent |
Analyte(s) |
Applications |
Sample Preparation |
Analytical Technique |
LODs |
EF 1 |
Reference |
|---|---|---|---|---|---|---|---|
|
G |
Co(II), Ni(II) |
Tap, river and sea water |
SPE |
FAAS |
0.36, 0.51 μg/L |
200 |
[59] |
|
GO-silica |
Mn(II), Co(II), Ni(II), Cu(II), Cd(II), Pb(II) |
Well, pond and lake water |
HF-SPME |
ICP-MS |
7.5, 0.39, 20, 23, 6.7, 28 ng/L |
10 |
[60] |
|
G |
Sulfonylurea herbicides |
Environmental water samples |
SPE |
UHPLC-MS |
0.28–0.53 ng/L |
N/R 2 |
[61] |
|
GO |
Mn(II), Fe(III) |
Tap, mineral, river water |
SPE |
FAAS |
0.145, 0.162 μg/L |
325 |
[62] |
|
GO-silica |
Cu(II), Pb(II) |
Mineral, waste and sea water |
SPE |
FAAS |
0.084, 0.27 μg/L |
200–250 |
[63] |
|
GO-EDA |
Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Pb(II) |
Waste water from industry |
d-μSPE |
ED-XRF |
0.07, 0.10, 0.07, 0.08, 0.06, 0.10 μg/L |
N/R |
[64] |
|
GO |
Co(II), Ni(II), Cu(II), Zn(II), Pb(II) |
Waste water from industry |
d-μSPE |
ED-XRF |
0.5, 0.7, 1.8, 1.5, 1.4 μg/L |
N/R |
[65] |
|
GO |
Co(II), Ni(II) |
Mineral and spring water |
SPE |
FAAS |
0.25, 0.18 μg/L |
250 |
[66] |
|
GO |
Cr(III), Co(II), Ni(II), Cu(II), Zn(II), Pb(II) |
Environmental water samples |
d-μSPE |
ED-XRF |
0.07–0.25 μg/L |
N/R |
[67] |
|
GO-Gly |
Cr(III), Cu(II), Zn(II) |
Tap, river, estuarine and lake water |
d-μSPE |
ED-XRF |
0.15, 0.07, 0.08 μg/L |
1575, 890, 810 |
[68] |
|
GO-S |
Cu(II), Zn(II), Pb(II), Cr(III) |
Lake, river, mineral, spring and sea water |
d-μSPE |
ED-XRF |
0.06–0.10 μg/L |
520–3120 |
[69] |
|
GO |
Pb(II), Cd(II), Cr(III) |
River water |
CHd-μSPE |
ETAAS |
0.035, 0.005, 0.012 μg/L |
14.7, 16.1, 15.4 |
[70] |
|
GO-MWCNT |
Cr(III), Fe(III), Pb(II), Mn(II) |
Wastewater |
SPE |
ICP-OES |
0.16, 0.50, 0.24, 0.38 μg/L |
75 |
[71] |
|
GMeS |
Sulfonamides |
Lake water |
SPE |
HPLC |
0.10–0.29 μg/kg 3 |
96–99 |
[72] |
|
GO-TTC |
Pb(II), Cu(II) |
Sea water |
SPE |
FAAS |
0.32, 0.13 μg/L |
83.3 |
[73] |
|
GO-sponges |
Methylene blue |
Wastewater |
N/R |
UV-vis |
N/R |
N/R |
[74] |
|
GO-MMT/SA |
Methylene blue |
Wastewater |
N/R |
UV-vis |
N/R |
N/R |
[75] |
1 EF = Enrichment Factor., 2 N/R = Not Reported., 3 Only the Limit of Quantification (LOQ) was reported.
Table 2. Main applications of magnetic graphene-based nanomaterials in environmental samples analysis.
|
Adsorbent |
Analyte(s) |
Applications |
Sample Preparation |
Analytical Technique |
LODs |
EF |
Reference |
|---|---|---|---|---|---|---|---|
|
mGO |
PAHs |
Tap, river, sea water |
MSPE |
HPLC-UV |
0.09–0.19 μg/L |
25 |
[76] |
|
mGO |
PCB 28 |
School sewage, river water |
MSPE |
GC-MS |
0.027–0.059 μg/L |
200 |
[77] |
|
mGO |
Sulfonamides |
Tap, river water |
MSPE |
HPLC-DAD |
0.05–0.10 mg/L |
N/R |
[78] |
|
mGO-porphyrin |
Sulfonamides |
Tap, river water |
MSPE |
HPLC-DAD |
0.2 mg/L |
N/R |
[79] |
|
GO-MC-MTPS |
Hg(II) |
Tap, sea water |
MSPE |
CV-AAS 1 |
0.06 μg/L |
80 |
[80] |
|
mGO-DETA |
Cd(II), Pb(II) |
Sea, river, well water |
MSPE |
FAAS |
0.40, 0.38 μg/L |
150, 167 |
[81] |
|
mGO |
Cr(III), Cr(VI), Au(III) |
Drinking, river, spring, sea, waste water |
d-μSPE |
FAAS |
0.1, 0.1, 0.004 μg/L |
200, 200, 500 |
|
|
mGO |
Au(III) |
Tap, lake, sea water |
dSPE |
MP-AES |
5 ng/L |
60 |
[84] |
|
TETA-mGO |
Phenolic estrogens |
Tap, river, well water |
MSPE |
UFLC-MS/MS |
0.15–1.5 ng/L |
10,000 |
[85] |
|
mGO-DVB-VA |
Pb(II), Cd(II), Cu(II), Ni(II), Co(II) |
Waste water |
MSPE |
FAAS |
0.37–2.39 μg/L |
40 |
[86] |
|
mf-GO |
Cr(III), Cr(VI) |
River, tannery and electroplating waste water |
d-MSPE |
FAAS |
5.2, 1.6 μg/L |
10 |
[87] |
|
mGO |
Imatinib, Doxorubicin |
Well, waste water |
MSPE |
HPLC-UV |
1.9, 1.8 μg/L |
N/R |
[88] |
|
mGO |
2,4,6-trinitrotoluene |
Reservoir, drinking, waste water |
MSPE |
HPLC-UV |
0.3 μg/L |
153 |
[89] |
|
mGO-MBT 2 |
Cd(II), Cu(II), Pb(II) |
Tap, lake, sea water |
MSPE |
FAAS |
0.19, 0.35, 0.24 μg/L |
400 |
[90] |
|
mGO-HQ 3 |
Cd(II), Pb(II) |
Water samples |
MSPE |
FAAS |
0.09, 0.27 μg/L |
130.43 |
[91] |
|
mGO/SiO2@coPPy-Th |
Cu(II), Cr(III), Zn(II), Cd(II), Pb(II) |
Well, river, bottled mineral water |
MSPE |
FAAS |
0.15–0.65 μg/L |
36–44 |
[92] |
|
mGO-MBT |
Au(III), Pd(II), Ag(I) |
Waste water |
MSPE |
FI-ICP-OES |
45–76 ng/L |
160, 160, 140 |
[93] |
|
mGO-imidazolium |
Cr(III), Cr(VI) |
Waste water |
MSPE |
ETAAS |
1.9 ng/L |
357 |
[94] |
|
mGO |
di-2-ethylhexyl phthalate |
Water samples |
MSPE |
HPLC-DAD |
0.35 μg/L |
100 |
[95] |
1 Cold Vapor Atomic Absorption Spectrometry, 2 Magnetic Graphene Oxide modified with 2-mercaptobenzothiazole, 3 Magnetic Graphene Oxide modified with 8-hyrdoxyquinolone.
This entry is adapted from the peer-reviewed paper 10.3390/app11073028
References
- Chen, Y.; Xia, L.; Liang, R.; Lu, Z.; Li, L.; Huo, B.; Li, G.; Hu, Y. Advanced materials for sample preparation in recent decade. TrAC Trends Anal. Chem. 2019, 120, 115652.
- Plastiras, O.-E.; Andreasidou, E.; Samanidou, V. Microextraction Techniques with Deep Eutectic Solvents. Molecules 2020, 25, 6026.
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. TrAC Trends Anal. Chem. 2020, 127, 115882.
- Xia, S.; Dong, J.; Chen, Y.; Wang, Y.; Chen, X. Three dimensional phytic acid-induced graphene as a solid-phase microextraction fiber coating and its analytical applications for nerolidol in tea. Chin. Chem. Lett. 2018, 29, 107–110.
- Jiang, H.-L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.-S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120.
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/ nanocomposite. Microchim. Acta 2018, 185, 1–9.
- Yap, P.L.; Auyoong, Y.L.; Hassan, K.; Farivar, F.; Tran, D.N.; Ma, J.; Losic, D. Multithiol functionalized graphene bio-sponge via photoinitiated thiol-ene click chemistry for efficient heavy metal ions adsorption. Chem. Eng. J. 2020, 395, 124965.
- Maciel, E.V.S.; de Toffoli, A.L.; Neto, E.S.; Nazario, C.E.D.; Lanças, F.M. New materials in sample preparation: Recent advances and future trends. TrAC Trends Anal. Chem. 2019, 119, 115633.
- Shivakumar, R.; Bolker, A.; Tsang, S.H.; Atar, N.; Verker, R.; Gouzman, I.; Hala, M.; Moshe, N.; Jones, A.; Grossman, E.; et al. POSS enhanced 3D graphene—Polyimide film for atomic oxygen endurance in Low Earth Orbit space environment. Polymer 2020, 191, 122270.
- Kyzas, G.Z.; Travlou, N.A.; Deliyanni, E.A. The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surf. B Biointerfaces 2014, 113, 467–476.
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205.
- Sengupta, J.; Hussain, C.M. Graphene and its derivatives for Analytical Lab on Chip platforms. TrAC Trends Anal. Chem. 2019, 114, 326–337.
- Nayl, A.; Abd-Elhamid, A.; El-Shanshory, A.A.; Soliman, H.M.; Kenawy, E.-R.; Aly, H. Development of sponge/graphene oxide composite as eco-friendly filter to remove methylene blue from aqueous media. Appl. Surf. Sci. 2019, 496, 143676.
- Gazzari, S.; Cortés-Arriagada, D. Interaction of trivalent arsenic on different topologies of Fe-doped graphene nanosheets at water environments: A computational study. J. Mol. Liq. 2019, 289, 1–7.
- Zhang, L.; Zhang, Y.; Tang, Y.; Li, X.; Zhang, X.; Li, C.; Xu, S. Magnetic solid-phase extraction based on Fe3O4/graphene oxide nanoparticles for the determination of malachite green and crystal violet in environmental water samples by HPLC. Int. J. Environ. Anal. Chem. 2018, 98, 215–228.
- Martín, A.; Escarpa, A. Graphene: The cutting–edge interaction between chemistry and electrochemistry. TrAC Trends Anal. Chem. 2014, 56, 13–26.
- Mateos, R.; Vera, S.; Díez-Pascual, A.M.; Andrés, M.P.S. Graphene solid phase extraction (SPE) of synthetic antioxidants in complex food matrices. J. Food Compos. Anal. 2017, 62, 223–230.
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064.
- Yang, M.; Tian, H.; Zhu, J.; He, J. Graphene Nanomaterials in Energy and Environment Applications. Handb. Graphene 2019, 5, 1–25.
- Qi, C.; Zhao, L.; Lin, Y.; Wu, D. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J. Colloid Interface Sci. 2018, 517, 18–27.
- Zhou, S.; Yao, W.; Wang, Z.; Ma, L.; Lu, Z.; Hou, C. The first-principles calculations to explore the mechanism of oxygen diffusion on vacancy defective graphene in marine environment. Appl. Surf. Sci. 2020, 525, 146585.
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Proc. Eng. 2017, 184, 469–477.
- Maggira, M.; Deliyanni, E.A.; Samanidou, V.F. Synthesis of Graphene Oxide Based Sponges and Their Study as Sorbents for Sample Preparation of Cow Milk Prior to HPLC Determination of Sulfonamides. Molecules 2019, 24, 2086.
- García-Mesa, J.; Leal, P.M.; Guerrero, M.L.; Alonso, E.V. Simultaneous determination of noble metals, Sb and Hg by magnetic solid phase extraction on line ICP OES based on a new functionalized magnetic graphene oxide. Microchem. J. 2019, 150, 104141.
- Shahabi, M.; Raissi, H. Comprehensive theoretical prediction of the dynamics and stability properties of Tegafur pharmaceutical agent on the Graphene based nanostructures in aqueous environment. Appl. Surf. Sci. 2018, 455, 32–36.
- Li, W.-K.; Shi, Y.-P. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2019, 118, 652–665.
- Jon, C.-S.; Meng, L.-Y.; Li, D. Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. TrAC Trends Anal. Chem. 2019, 120, 115641.
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796.
- Yao, Y.; Ping, J. Recent advances in graphene-based freestanding paper-like materials for sensing applications. TrAC Trends Anal. Chem. 2018, 105, 75–88.
- Wang, Z.; Han, Q.; Xia, J.; Xia, L.; Ding, M.; Tang, J. Graphene-based solid-phase extraction disk for fast separation and preconcentration of trace polycyclic aromatic hydrocarbons from environmental water samples. J. Sep. Sci. 2013, 36, 1834–1842.
- Núñez, C.; Triviño, J.J.; Segura, R.; Arancibia, V. Development of a fast and sensitive method for the determination of As(III) at trace levels in urine by differential pulse anodic voltammetry using a simple graphene screen–printed electrode. Microchem. J. 2020, 159, 105393.
- Wang, Z.; Yao, M.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Influence of reaction media on synthesis of dialdehyde cellulose/GO composites and their adsorption performances on heavy metals. Carbohydr. Polym. 2019, 232, 115781.
- Zhang, Y.; Guan, J.; Wu, J.; Ding, S.; Yang, J.; Zhang, J.; Dong, A.; Deng, L. N-alkylated chitosan/graphene oxide porous sponge for rapid and effective hemostasis in emergency situations. Carbohydr. Polym. 2019, 219, 405–413.
- Wu, J.; Chen, L.; Mao, P.; Lu, Y.; Wang, H. Determination of chloramphenicol in aquatic products by graphene-based SPE coupled with HPLC-MS/MS. J. Sep. Sci. 2012, 35, 3586–3592.
- Service, R.F. Carbon Sheets an Atom Thick Give Rise to Graphene Dreams. Science 2009, 324, 875–877.
- Hou, X.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC Trends Anal. Chem. 2019, 119, 115603.
- Sadegh, H. Development of graphene oxide from graphite: A review on synthesis, characterization and its application in wastewater treatment. Rev. Adv. Mater. Sci. 2017, 49, 38–43.
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020.
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069.
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered graphene oxide membranes for water treatment: A review. Carbon 2018, 139, 964–981.
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal. Chem. 2013, 51, 33–43.
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Wang, X.; Liu, B.; Lu, Q.; Qu, Q. Graphene-based materials: Fabrication and application for adsorption in analytical chemistry. J. Chromatogr. A 2014, 1362, 1–15.
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546.
- Valcárcel, M.; Cárdenas, S.; Simonet, B.; Moliner-Martínez, Y.; Lucena, R. Carbon nanostructures as sorbent materials in analytical processes. TrAC Trends Anal. Chem. 2008, 27, 34–43.
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446.
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B Biointerfaces 2019, 173, 698–708.
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal. Chem. 2014, 59, 50–58.
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248.
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic graphene oxide: Effect of preparation route on reactive black 5 adsorption. Materials 2013, 6, 1360–1376.
- Kluchova, K.; Zboril, R.; Tucek, J.; Pecova, M.; Zajoncova, L.; Safarik, I.; Mashlan, M.; Markova, I.; Jancik, D.; Sebela, M.; et al. Superparamagnetic maghemite nanoparticles from solid-state synthesis—Their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials 2009, 30, 2855–2863.
- Zheng, Y.H.; Cheng, Y.; Bao, F.; Wang, Y.S. Synthesis and magnetic properties of Fe3O4 nanoparticles. Mater. Res. Bull. 2006, 41, 525–529.
- Li, C.; Wei, Y.; Liivat, A.; Zhu, Y.; Zhu, J. Microwave-solvothermal synthesis of Fe3O4 magnetic nanoparticles. Mater. Lett. 2013, 107, 23–26.
- Deng, H.; Huang, J.; Qin, C.; Xu, T.; Ni, H.; Ye, P. Preparation of high-performance nanocomposite membranes with hydroxylated graphene and graphene oxide. J. Water Process. Eng. 2021, 40, 101945.
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211.
- Arshad, F.; Selvaraj, M.; Banat, F.; Abu Haija, M. Removal of metal ions and organics from real refinery wastewater using double- functionalized graphene oxide in alginate beads. J. Water Process. Eng. 2020, 38, 101635.
- Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules 2020, 25, 2411.
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148.
- Wang, Y.-K.; Gao, S.-T.; Ma, J.-J.; Li, J.-C. Application of Graphene as a Sorbent for Simultaneous Preconcentration and Determination of Trace Amounts of Cobalt and Nickel in Environmental Water and Vegetable Samples. J. Chin. Chem. Soc. 2012, 59, 1468–1477.
- Su, S.; Chen, B.; He, M.; Hu, B. Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 2014, 123, 1–9.
- Li, C.; Lu, A.; Wang, J.; Li, J.; Ping, H.; Luan, Y.; Chen, J.; Ha, X. Determination of five sulfonylurea herbicides in environmental waters and soil by ultra- high performance liquid chromatography with tandem mass spectrometry after extraction using graphene. J. Sep. Sci. 2014, 37, 3714–3721.
- Pourjavid, M.R.; Sehat, A.A.; Arabieh, M.; Yousefi, S.R.; Hosseini, M.H.; Rezaee, M. Column solid phase extraction and flame atomic absorption spectrometric determination of manganese(II) and iron(III) ions in water, food and biological samples using 3-(1-methyl-1H-pyrrol-2-yl)-1H-pyrazole-5-carboxylic acid on synthesized graphene oxide. Mater. Sci. Eng. C 2014, 35, 370–378.
- Sitko, R.; Zawisza, B.; Talik, E.; Janik, P.; Osoba, G.; Feist, B.; Malicka, E. Spherical silica particles decorated with graphene oxide nanosheets as a new sorbent in inorganic trace analysis. Anal. Chim. Acta 2014, 834, 22–29.
- Zawisza, B.; Baranik, A.; Malicka, E.; Talik, E.; Sitko, R. Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim. Acta 2016, 183, 231–240.
- Zawisza, B.; Sitko, R.; Malicka, E.; Talik, E. Graphene oxide as a solid sorbent for the preconcentration of cobalt, nickel, copper, zinc and lead prior to determination by energy- dispersive X-ray fluorescence spectrometry. Anal. Methods 2013.
- Pourjavid, M.R.; Arabieh, M.; Yousefi, S.R.; Jamali, M.R.; Rezaee, M.; Hosseini, M.H.; Sehat, A.A. Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real samples. Mater. Sci. Eng. C 2015, 47, 114–122.
- Pytlakowska, K. Dispersive micro solid-phase extraction of heavy metals as their complexes with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol using graphene oxide nanoparticles. Microchim. Acta 2016, 183, 91–99.
- Pytlakowska, K.; Kozik, V.; Matussek, M.; Pilch, M.; Hachuła, B.; Kocot, K. Glycine modified graphene oxide as a novel sorbent for preconcentration of chromium, copper, and zinc ions from water samples prior to energy dispersive X-ray fluorescence spectrometric determination. RSC Adv. 2016, 6, 42836–42844.
- Pytlakowska, K.; Pilch, M.; Hachuła, B.; Nycz, J.E.; Kornaus, K.; Pisarski, W.A. Energy dispersive X-ray fluorescence spectrometric determination of copper, zinc, lead and chromium species after preconcentration on graphene oxide chemically modified with mercapto-groups. J. Anal. At. Spectrom. 2019, 34, 1416–1425.
- Ghazaghi, M.; Mousavi, H.Z.; Rashidi, A.M.; Shirkhanloo, H.; Rahighi, R. Innovative separation and preconcentration technique of coagulating homogenous dispersive micro solid phase extraction exploiting graphene oxide nanosheets. Anal. Chim. Acta 2016, 902, 33–42.
- Zhu, X.; Cui, Y.; Chang, X.; Wang, H. Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) Ions in wastewater using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta 2016, 146, 358–363.
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8.
- Mosavi, S.S.; Ghanemi, K.; Nickpour, Y. Graphene oxide nanosheets modified with trithiocyanuric acid for extraction and enrichment of Pb (II) and Cu (II) ions in seawater. Water Environ. J. 2018, 32, 377–383.
- Sun, Y.; Chen, L.; Yu, J.; Yoon, B.; Lee, S.K.; Nam, J.-D.; Ci, L.; Suhr, J. Lightweight graphene oxide-based sponges with high compressibility and durability for dye adsorption. Carbon 2020, 160, 54–63.
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833.
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395.
- Zeng, S.; Gan, N.; Weideman-Mera, R.; Cao, Y.; Li, T.; Sang, W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115.
- Shi, P.; Ye, N. Magnetite–graphene oxide composites as a magnetic solid-phase extraction adsorbent for the determination of trace sulfonamides in water samples. Anal. Methods 2014, 6, 9725–9730.
- Shi, P.; Ye, N. Investigation of the adsorption mechanism and preconcentration of sulfonamides using a porphyrin-functionalized Fe3O4-graphene oxide nanocomposite. Talanta 2015, 143, 219–225.
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56.
- Aliyari, E.; Alvand, M.; Shemirani, F. Simultaneous separation and preconcentration of lead and cadmium from water and vegetable samples using a diethylenetriamine-modified magnetic graphene oxide nanocomposite. Anal. Methods 2015, 7, 7582–7589.
- Kazemi, E.; Dadfarnia, S.; Shabani, A.M.H. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278.
- Kazemi, E.; Shabani, A.M.H.; Dadfarnia, S.; Izadi, F. Speciation and determination of chromium ions by dispersive micro solid phase extraction using magnetic graphene oxide followed by flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2017, 97, 1080–1093.
- Ahmad, H.; Jalil, A.A.; Triwahyono, S. Dispersive solid phase extraction of gold with magnetite-graphene oxide prior to its determination via microwave plasma-atomic emission spectrometry. RSC Adv. 2016, 6, 88110–88116.
- Chen, X.-H.; Pan, S.-D.; Ye, M.-J.; Li, X.-P.; Zhao, Y.-G.; Jin, M.-C. Magnetic solid-phase extraction based on a triethylenetetramine-functionalized magnetic graphene oxide composite for the detection of ten trace phenolic environmental estrogens in environmental water. J. Sep. Sci. 2016, 39, 762–768.
- Khan, M.; Yilmaz, E.; Sevinc, B.; Sahmetlioglu, E.; Shah, J.; Jan, M.R.; Soylak, M. Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta 2016, 146, 130–137.
- Islam, A.; Ahmad, H.; Zaidi, N.; Kumar, S. A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim. Acta 2016, 183, 289–296.
- Arvand, M.; Masouleh, A.N. Magnetic solid-phase extraction of imatinib and doxorubicin as cytostatic drugs by Fe3O4/graphene oxide nanocomposite. J. Iran. Chem. Soc. 2017, 14, 1673–1682.
- Dos Reis, L.C.; Vidal, L.; Canals, A. Graphene oxide/Fe3O4 as sorbent for magnetic solid-phase extraction coupled with liquid chromatography to determine 2,4,6-trinitrotoluene in water samples. Anal. Bioanal. Chem. 2017, 409, 2665–2674.
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. Trace amounts of Cd(II), Cu(II) and Pb(II) ions monitoring using Fe3O4 @graphene oxide nanocomposite modified via 2-mercaptobenzothiazole as a novel and efficient nanosorbent. J. Mol. Liq. 2017, 231, 386–395.
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. Synthesis and Application of Magnetic Graphene Oxide Modified with 8-Hydroxyquinoline for Extraction and Preconcentration of Trace Heavy Metal Ions. ChemistrySelect 2017, 2, 1282–1289.
- Molaei, K.; Bagheri, H.; Asgharinezhad, A.A.; Ebrahimzadeh, H.; Shamsipur, M. SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 2017, 167, 607–616.
- Neyestani, M.R.; Shemirani, F.; Mozaffari, S.; Alvand, M. A magnetized graphene oxide modified with 2-mercaptobenzothiazole as a selective nanosorbent for magnetic solid phase extraction of gold(III), palladium(II) and silver(I). Microchim. Acta 2017, 184, 2871–2879.
- Sarikhani, Z.; Manoochehri, M. Determination of Ultra Trace Cr(III) and Cr(VI) Species by Electrothermal Atomic Absorption Spectrometry after Simultaneous Magnetic Solid Phase Extraction with the Aid of a Novel Imidazolium-Functionalized Magnetite Graphene Oxide Nanocomposite. Bull. Chem. Soc. Jpn. 2017, 90, 746–753.
- Ma, L.; Huang, J.; Zhou, M. Magnetic graphene oxide for efficient solid phase extraction of DEHP. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544.
