Magnetic nanoparticles gained considerable attention in last few years due to their remarkable properties. Superparamaganetism, non-toxicity, biocompatibility, chemical inertness, and environmental friendliness are some of the properties that make iron oxide nanoparticles (IONPs) an ideal choice for biomedical applications. Along with being easily tuneable and a tailored surface for conjugation of IONPs, their physio-chemical and biological properties can also be varied by modifying the basic parameters for synthesis that enhances the additional possibilities for designing novel magnetic nanomaterial for theranostic applications.
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Early diagnosis and therapy of crucial diseases are pertinent for accurate diagnosis in a timely manner. Nanotechnology-based approaches paved the way for the diagnosis of various diseases. Improvement in the sensitivity and accuracy of diagnosis paved the way for efficient and early diagnosis. Various non-invasive in-vivo imaging techniques were developed for the diagnosis to visualize the progression of abnormal cells by binding to the target site. These non-invasive techniques include magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), computed tomography (CT), nuclear imaging of positron emission tomography (PET), and optical (or fluorescence) imaging. The above said techniques are based on in-vivo imaging that assist the clinicians to locate the physiological and anatomical state of the organs.
IONPs majorly are used as a contrast agent in imaging, targeting, diagnostics, and treatment of hyperthermia. Magnetite (Fe
3O
4), maghemite (γ- Fe
2O
3) and hematite (α- Fe
2O
3) are some of the important examples of SPIONs [
1]. The presence of Fe
2+ and Fe
3+ ions make SPIONs ideal candidate for biomedical applications. Superparamagnetic iron oxide nanoparticles (SPIONs) consisted of a ferrimagnetic group of the magnetic particles with major applications in biomedicine and bioengineering. Currently, SPIONs based therapeutics are at clinical, and preclinical trials, and few already reached to the market [
2]. However, cellular toxicity occurred by SPIONs could potentially lead to adverse effects such as apoptosis, DNA damage, reactive oxygen species (ROS) generation, inflammation, mitochondrial function impairment, and chromosome condensation.
Polymeric materials such as polyethylene glycol (PEG), starch, and dextran polymers were widely used for the coating of SPIONs due to less toxicity, escaped by macrophages or spleen cells [
3]. Advancement in the field of stimuli responsive polymers has occurred, due to a reversible phase transition upon exposure to environmental changes such as temperature, pH, light, enzyme, and the magnetic field [
4]. The hydrophilic capsule of lipids due to its amphiphillic properties used for the coating of SPIONs includes various methods such as co-precipitation, thermal decomposition, sol-gel, microemulsion, electrochemical, and biosynthesis [
5,
6,
7]. The present review aims to provide an insight into biomedical and clinical applications such as Magnetic Resonance Imaging (MRI), Magnetic Particle Imaging (MPI), Computed Tomography (CT), Positron Emission Tomography (PET), nanozyme applications for biosensing, gene and drug delivery, and hyperthermia and photothermal therapy, and broad spectrum antimicrobial applications in diagnosis of various diseases.
2. Biomedical and Clinical Applications
2.1. Magnetic Resonance Imaging (MRI)
MRI is an imaging technique that employs a strong magnetic field and radio waves for the creation of high-resolution images of various organs in the human and animal body. It has been used very widely in clinical radiology that uses non-ionizing radiation in experimental settings. Additionally, the modality is tomographic with the penetration of soft tissue with high resolution [
4,
8].
MRI utilizes the magnetic field, radio waves, or electric fields to illustrate the detailed internal structure of the body. MRI contrast agents can be divided into two categories, T1 and T2 agents. T1 agents alter the longitudinal relaxation time of water protons whereas T2 agents alter the transverse relaxation time of water protons. Positive T1—weighted, it shortens the T1 and has a moderate effect on T2 providing a brighter image. On the contrary negative contrast of T2/T2* weighted and shortens the T2 relaxation time and leads to the formation of dark images [
9]. Tracking and monitoring of treatment, delivery of cells into the body, its proliferation, diffusion time, and migration rate require in-vivo imaging of the cells. Various imaging techniques are being used for this purpose, such as positron emission tomography (PET), single positron emission computed tomography (SPECT), X-ray based computed tomography (CT), and magnetic resonance imaging (MRI). Among all, MRI has shown to have a high spatial resolution up to 100 µm, in the absence of exposure to ionizing radiation [
8], which makes it a great imaging technique for in-vivo cell imaging.
SPIONs are extensively used as contrast agents due to their superparamagnetic properties, biocompatibility, and low cost. SPIONs are used for visualizing tumor and metastatic cancer in liver, spleen, lymph nodes, and as a blood pool agent for angiography as well for inflammatory lesions. The nanoparticles reduce the relaxation time of the surrounding protons due to their superparamagnetic properties, which makes them suitable candidates as MRI contrast agents. IONPs based contrast agents were chosen over gadolinium-based contrast agents due to their low toxicity [
10]. Ferucarbotran Resovist by Bayer Schering Pharma AG, contrast the effect of T1/T2, but most of their negative contrast and approved in few countries such as Japan, EU, and Australia [
11]. Ferumoxytol (Feraheme) IONPs are approved by the FDA for the treatment of iron deficiency in patients suffering from chronic kidney diseases [
12,
13].
2.2. Magnetic Particle Imaging (MPI)
MPI is a tomographic technique that is non-invasive and detects tracers particles with superparamagnetic properties. MPI has potential applications in the field of diagnostics, imaging, and materials properties. MPI can be used to locate and measure the concentration of nanoparticles with non-ionizing radiation at any depth within the body to produce a signal. MPI signals are directly generated from the superparamagnetic nanoparticles when magnetized, which is 10
7 times more sensitive than MRI signals. Magnetic particle spectrometer (MPS) has the potential for 3D in-vivo imaging with high special resolution where SPIONs were employed for lactoferrin conjugation. The MPS signal was recorded using a custom-built magnetic particle spectrometer (MPS) [
14,
15]. Overexpressed cancer cells secrete specific proteases, such as trypsin, matrix metalloprotease-2 (MMP-2), which was detected via MPS. Neutravidin-coated SPIONs were aggregated in the presence of biotin labeled peptides that were later selectively identified and cleaved by specific proteases which was present in the peptides (protease cleavage site) which led to the dispersion of the SPIONs. The aggregation or dispersion state of the SPIONs were identified by MPS signals through magnetic relaxation characteristics. Hence, MPS can be used in biomedical applications such as rapid detection of proteases in biological samples for example blood, urine, tissue extracts, and cell culture media for diagnosis of various types of cancer [
16].
2.3. Computed Tomography (CT)
A computed tomography can be used in radiology for medical imaging to acquire detailed images of the body in a non-invasive manner. A CT scan measures X-ray attenuations of different tissues inside the body with rotating x-ray tube and detectors. The X-ray measurements usually taken multiple times from different angles to process on a computer to reconstruct the algorithms for the generation of tomographic images of a body. Contrast agents for CT are injected intravenously, the widely used agents are iodine conjugated small particles, and gadolinium-based contrast agents. However, patients with renal impairment are reported to be highly sensitive to iodine-based contrast agents, and gadolinium-based agents show toxicity in cell or tissues. Therefore, iron-oxide-based nanoparticles were taken into consideration as an alternate of iodine-based contrast agents [
17,
18].
Gold-coated iron oxide glycol nanoparticles can be used as an effective contrast agent for imaging in CT. It was confirmed that small size magnetic nanoparticles are highly biocompatible, biodegradable, and possessive of X-ray attenuation characteristics, and represent low toxicity over a longer period of time. These characteristics make them a strong candidate for both CT and MRI imaging [
19,
20]. Naha et al. synthesized a nanocomposite of bismuth-IONPs with dextran coating to check the cytotoxicity, accumulation, and half-life of the nanoconjugate. Lower cytotoxicity was observed in HepG2 (Human liver cancer cell line), and CT imaging was done after overnight incubation with nanoparticles to observe the contrast in blood vessels and heart. The nanoconjugate was found to have a protracted circulation half-life in the body. It was established that the nanoconjugate had a strong X-ray attenuation property and, biocompatibility that make it a good candidate as contrast agent in CT and MRI [
19].
Reguera et al. designed and synthesized gold-iron-oxid- based Janus magnetic-plasmonic nanoparticles, which were used as a contrast agent for numerous techniques such as CT (Computed tomography), MRI (magnetic resonance imaging), TEM (transmission electron microscopy), PAI (photo acoustic imaging), SERS (surface enhanced Raman spectroscopy), optical imaging. Hence, by using these multifunctional nanoparticle agents, maximum cellular information can be obtained at once making them serve as great imaging tool in biomedical platform [
21]. However, the adverse effects caused by the use of ionizing radiations sometimes restrict use in the patients.
2.4. PET (Positron Emission Tomography)
Positron emission tomography (PET) is an imaging technique that employs radioactive material known as radio-tracers to envision and measure changes in metabolic events. PET enables localization and quantification of activities in specific organs or tissues for whole body imaging; however, it cannot reveal about the anatomy or morphology of the tissues. Different tracers were used for imaging, based on the target such as 18F-FDG for cancer, NaF-F18 for bone formation, and oxygen-15 to measure blood flow. In PET, a radioisotope was attached to a drug, followed by injection into the body as a tracer. The emitted gamma rays were detected and used for the reconstruction of 3-D image. PET scanners can be combined with a CT scanner in the same session and are known as PET-CT scanners.
Torres et al. developed a novel contrast agent composed of IONPs (Feridex) labeled with a
64Cu based bi-functional chelator for in-vivo imaging of lymph node [
22]. Tri-modality agents were synthesized by conjugation of a PET tracer
64Cu with dextran coated magneto fluorescent nanoparticles for PET, MRI, and fluorescence imaging (). Tri-modality nanoparticles were used to detect macrophages in atherosclerotic patients [
23]. Stelter et al. functionalized IONPs with Ga-DTPA to synthesize a Ga-DTPA-IONP complex and injected into mouse model. PET and magnetic resonance imaging were performed to observe the accumulation of IONPs in kidney and spleen regions [
24]. Glaus et al. synthesized IONPs micelles, which were radioactively labeled by
64Cu using DOTA as a chelating agent. In-vivo biodistribution was observed after 24 h of injection in mice through PET and MRI with 143 min half-life of the radioactive nanoconjugate, leading to accumulation in hepatic and splenic region [
25]. Aryal et al. utilized
64Cu labeled PLGA functionalized SPIONs to locate breast cancer cells in a xenograft mouse tumor model. The nanohybrid was selected due to its longer circulation time as the nanohybrid was taken up by the tumor cells via EPR effect and located at the tumor region using PET and T2 weighed MRI [
26]. Some SPIONs are approved by US-FDA for imaging applications in clinical settings ().
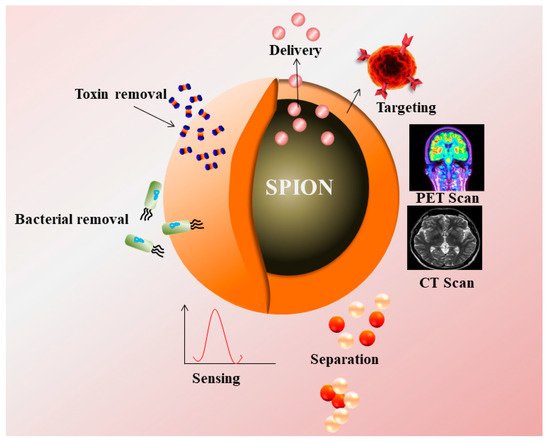
Figure 1. Superparamagnetic nanoparticles (SPIONs) as a contrast agent in PET, CT Scan, and their applications in targeting, drug delivery, removal of toxin, bacteria, and sensing.
Table 1. Clinically approved IONPs conjugated compounds and its applications at various biomedical platform.
| Serial No. |
Compound Name |
Coating |
Applications |
Clinically Approved |
References |
| 1 |
Ferumoxtran
(Combidex®) |
Dextran |
Lymph node imaging, macrophage imaging, blood pool agent, cell labelling, CNS imaging, MRI |
In clinical trials |
[27,28] |
| 2 |
Ferucarbotran
(Resovist®) |
Carboxydextran |
Liver imaging, cell labelling, CNS imaging, MRI |
Approved |
[29,30,31] |
| 3 |
Ferumoxide
(Feridex®) |
Dextran |
Liver imaging, cell labelling, CNS imaging, MRI |
Withdrawn from market |
[32,33,34] |
| 4 |
Ferumoxytol
(Feraheme®) |
Carboxymethyl-dextran |
Iron replacement therapy in patients with chronic kidney diseases |
Approved |
[35] |
| 5 |
Feruglose
(Clariscan™) |
PEGylated starch |
Blood pool agent, MRI |
In clinical trials |
[36] |
| 6 |
Ferumoxsil
(Gastromark®) |
Siloxane |
Oral GI imaging |
Approved |
[13,37] |
2.5. Nanozyme Applications for Biosensing
Nanozymes are the nanoparticles that exhibit the properties of enzymes such as oxidase, peroxidase, catalase, and superoxide dismutase. Various metals as well as metal oxides such as gold, silver, copper, SPIONs, and graphene nanosheets have shown HRP (horse radish peroxidase) like activities, which are currently being used for fabrication of biosensors. These non-enzymatic biosensors were used for detection of cholesterol concentration, creatinine, glucose, glutathione, H
2O
2, and urea. These biomolecules acted as biomarkers for various diseases, and biosensors developed for these molecules were used for early diagnosis of the diseases [
38,
39,
40,
41,
42]. Studies showed that coating SPIONs with anionic SPIONs showed a higher affinity for TMB (3,3′,5,5′-tetramethylbenzidine), and cationic SPIONs for ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) and OPD (O-phenylenediamine). Compared to natural enzymes, the nanozymes have various disadvantages such as slow substrate affinity and specificity. Therefore, novel methods for coating were used to enhance the catalytic activities of the nanozymes. Qian et al. synthesized nanozymes via conjugation of reduced graphene oxide on Fe
3O
4 nanospheres that was found to be stable for about three months when stored at 4 °C [
43].
Super paramagnetic iron oxide nanoparticles (SPIONs) have catalytic properties of peroxides and catalase, which play an important role in preventing the cellular oxidative damage. Magnetite based iron oxide nanoparticles (IONPs) mimic the peroxidase activity and are used for fabrication of peroxidase based biosensors. Unlike the peroxidase enzyme, these IONPs do not degrade at unfavorable temperatures and pH (). Kacar et al. developed an amperometric sensor for creatinine using Fe
3O
4 nanoparticles that were modified with carbon paste electrodes. This method required two catalytic reactions, creatinase and sarcosine oxidase, to produce H
2O
2. The sensor detected the presence of H
2O
2 to determine the creatine level. The presence of elevated levels of creatine in urine or blood indicated the renal abnormalities or failure [
44]. Gao et al. synthesized chitosan coated magnetic nanoparticles (CS-MNPs) for capture detection immunoassay, which detected the carcinoembryonic antigen (CEA) with a limit of detection (LOD) up to 1 ng/mL via sandwich ELISA, and direct ELISA [
45]. Currently, various pathogens were detected by novel immunoassays such as IgG,
Mycoplasma pneumonia,
Vibrio cholera, Rotavirus, Hepatocellular Carcinoma biomarker, Golgi protein 73 (GP73) [
46], Human Chorionic Gonadotropin (HCG), and cancer cells with Human Epidermal Growth Factor Receptor 2 (HER2) and Epidermal Growth Factor Receptor (EGFR) [
47]. Peter et al. developed positively charged nanoparticles and monitored their localization in-situ by optical biosensor and transmission electron microscope (TEM). The positively charged nanoparticles penetrated well into the cells as compared with negatively charged particles with an optimal size around 5 nm for the cellular uptake [
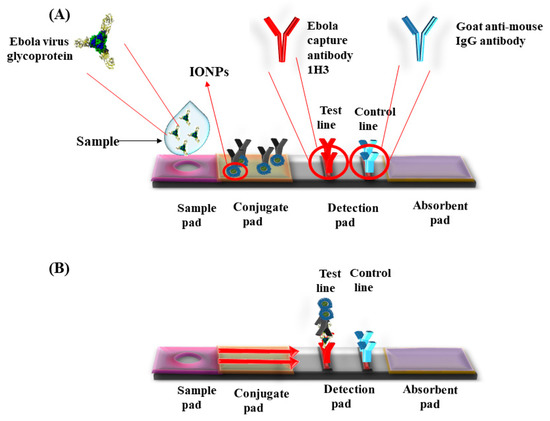
48]. A lateral flow assay was developed with IONPs’ nanozymes strip by Duan et al. for the detection of Ebola virus (EBOV). The nanostrip was capable to detect EBOV glycoprotein as low as 1 ng/mL (). The diagnostic capability was compared with ELISA that required less time to detect EBOV [
49].
Figure 2. Lateral flow dipstick device for the detection of Ebola virus using Iron oxide nanoparticles (IONPs). (A) Ebola capture antibody 1H3 coated on test line and control line coated with goat anti-mouse IgG antibody. Sample was applied in the sample well. (B) The sample runs via capillary action on the membrane. Presence of specific antigen for Ebola developed color in the detection pad.
Table 2. Chemically synthesized and modified IONPs nanozymes and its application for the detection of various biological compounds.
| Serial No. |
IONPs/Conjugated IONPs |
Applications |
References |
| 1 |
Fe3O4 NPs |
Exhibit peroxidase enzyme like activity. Used for fluorescent turn off system for detection of protein in urine |
[50] |
| 2 |
Chitosan coated IONPs with urease IONPs |
Used for detection of urea |
[51] |
| 3 |
IONPs |
Detection of Brucella antibodies with a LOD of 0.05 µg/mL |
[52] |
| 4 |
Fe3O4 nanocomposites/graphene oxide |
Biosensor synthesis for glucose detection with the range 0.5–10 mM |
[53] |
| 5 |
Fe3O4 NPs loaded in Co3O4 nanocages |
Used for glucose detection with the range of 0.5–30 µM with an LOD of 0.05 µM |
[54] |
This entry is adapted from the peer-reviewed paper 10.3390/cancers13092213