The circadian clock serves to coordinate metabolism and physiology with the diurnal cycles derived from the daily rotation of the earth. In Brassicaceae, circadian rhythms contribute to the temporal daily variation in diverse phytochemicals and, hence, to both resistance to biotic stress and edible crop health value. Evidence that levels of specialized metabolites present in Brassica crops can oscillate rhythmically during a day has begun to accumulate. Consistently, circadian clock also seems to play an essential role in the immunity timing coordination by ensuring appropriate chemical defenses in the right tissue and at the right time, controlling their biosynthesis, transport, and storage. In addition, it has been shown that circadian periodicity during the post-harvest period can also improve the longevity of tissue integrity and phytochemical content in diverse Brassica vegetables. Thus, temporal variation in metabolite concentrations can alter the accumulation of diverse phytochemicals and thereby the overall edible crop health value.
- circadian clock
- secondary metabolites
- clock-regulated metabolism
1. The Plant Circadian Clock
In plants, the primary rhythmic input is sunlight, which acts through photoreceptive proteins to reset the phase of the clock to local time. Circadian oscillations originate at the cellular level from the interactions of at least a dozen clock genes that interact through a series of transcriptional and post-transcriptional feedback loops to create further rhythmic gene expression [1][2]. Up to this point, the majority of work on the plant circadian oscillator has been conducted with the model plant Arabidopsis. Although several homologs of clock-related genes have been found to be conserved in Brassica crop species, not all the components have yet been identified, and the mechanistic details of signaling are only incompletely understood. Thus, in this section we first provide a general overview of the molecular mechanisms and clock network organization in the model plant Arabidopsis; then, we summarize the current knowledge of circadian oscillator in Brassica species.
1.1. The Arabidopsis Circadian Oscillator
In Arabidopsis, the circadian clock genes include over 20 transcription factors connected by an intricate network of feedback loops, including both activating and repressive components [3]. Core circadian clock genes are expressed throughout the day, but distinct morning, day, and evening transcriptional phases exist, and each phase represents the activity of multiple core circadian clock proteins (Figure 1). The core loop is comprised of two morning-expressed MYB transcription factors that have partially overlapping functions—CIRCADIANCLOCK-ASSOCIATED1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY); an evening-expressed gene, TIMING OF CAB EXPRESSION1 (TOC1); as well as a PSEUDO RESPONSE REGULATOR (PRR1) protein [4]. The expression of CCA1 and LHY peaks just after dawn, whereas the expression of TOC1 peaks in the early evening. CCA1 and LHY are members of the larger REVEILLE (RVE) gene family, which also contains the principal clock activators RVE8, RVE6, and RVE4 [5][6]. Furthermore, several other genes, including those codifying for additional PRRs proteins (PRR5, PRR7, and PRR9), GIGANTEA (GI), LUX ARRHYTHMO (LUX), BROTHER OF LUX ARRHYTHMO (NOX), as well as EARLY FLOWERING 3 (ELF3) and ELF4, are responsible for the generation of the morning and evening complex loops [5][7][8][9].
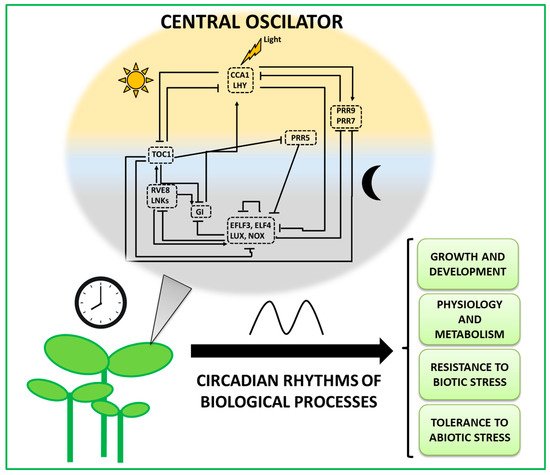
Figure 1. The plant circadian oscillator contributes to the regulation of multiple biological processes. The left area represents a simplified model of the regulatory interactions within the core circadian clock described in Arabidopsis. The clock components are represented from up to down according to the time of day of their peak expression. Yellow and gray backgrounds depict day and night, respectively. Rectangles denote functional groups, either because the components are members of the same gene family or because they act as a complex. Arrowheads and perpendicular lines illustrate the induction and repression of transcriptional activity, respectively. The right area region shows biological processes directly controlled by circadian clock genes or the rhythmic output of the circadian clock. For references and a complete description, please refer to the main text. CCA1, CIRCADIANCLOCK-ASSOCIATED1; LHY, LATE ELONGATED HYPOCOTYL; PRR9, PSEUDO RESPONSE REGULATOR9; PRR7, PSEUDO RESPONSE REGULATOR7; PRR5, PSEUDO RESPONSE REGULATOR5; EFLF3, EARLY FLOWERING3; ELF4, EARLY FLOWERING4; LUX, LUX ARRHYTHMO; NOX, BROTHER OF LUX ARRHYTHMO; GI, GIGANTEA; RVE8, REVEILLE8; LNKs, NIGHT LIGHT-INDUCIBLE AND CLOCK-REGULATED; TOC1, TIMING OF CAB EXPRESSION1.
During the morning, CCA1 and LHY repress the expression of evening-expressed genes, including TOC1, LUX, ELF3, and ELF4 [10][11], and, at the same time, promote the expression of PRR gene family members PRR9 and PRR7 [12]. The sequential expression of PRR genes over the course of the day imposes transcriptional repression on other PRR family members and additional core clock genes (for a review, see [13]). During the evening, the trimeric protein assembly composed of LUX, ELF3, and ELF4 suppresses the expression of PRR9, PRR7, GI, and NIGHT LIGHT-INDUCIBLE AND CLOCK-REGULATED1 (LNK1) in the late evening [9]. The evening complex represses itself near dawn through the inhibition of LUX, which allows the clock regulatory cycle to repeat the next day [14].
1.2. Circadian Clock Network in Brassica Crops
During the last few decades, much effort has been put into studying whether information of the Arabidopsis circadian clock can be extended to elucidate the molecular elements of the circadian core clock in Brassica crop species. The first studies began by identifying the natural allelic variation in clock function based on quantitative trait locus (QTL) analysis [15][16]. These studies investigated cotyledon movement from B. oleracea and B. rapa to detect genetic loci affecting the circadian period. Comparative genomic analysis based on collinearity between Brassica and Arabidopsis also allowed the identification of candidate genes known to regulate the period in Arabidopsis that may account for the additive circadian effects of specific QTL [17][18][19]. This includes gene families encoding PRRs, TOC1, CCA1, and LHY. Brassica species experienced an extra whole-genome triplication event compared with Arabidopsis. Therefore, as was expected, genes contributing to circadian clock function were retained in multiple copies in Bassica species. Song et al. [20] cloned and analyzed CCA1 genes from seven inbred lines and one cultivar of cabbage (Brassica oleracea). Two types of CCA1 alleles were detected and related to freezing-tolerant cabbage traits. In another study, the natural variation in CCA1 was associated with the flowering time in B. rapa and a high level of sequence variation was identified [21]. Genetic mapping and analysis of families of heterogeneous inbred lines showed that the natural variation in GI is responsible for a major quantitative trait locus in the circadian period in B. rapa. Loss-of-function mutations of GI from B. rapa and B. oleracea cultivars confer delayed flowering, perturbed circadian rhythms in leaf movement, caused leaf senescence, and increased freezing and salt tolerance, consistent with the effects of similar mutations in Arabidopsis [22][23].
More recently, Greenham et al. [24] and Kim et al. [25] performed high-resolution circadian transcriptome experiments to elucidate the B. rapa circadian network. They found that genes related to the clock displayed distinct phases, increasing or decreasing in regular patterns. In addition, the different copies of duplicated and triplicated genes did not necessarily all behave in the same way. Many of the copies had different rhythms, and some increased and decreased in patterns totally opposite to their counterparts. Not only did the daily patterns differ, but responses to stressors were also altered. Comparing these patterns to the patterns seen in Arabidopsis revealed that often, one B. rapa gene behaved just like its Arabidopsis equivalent, while its copies had evolved new behaviors. The authors conclude that different behaviors of the copies of each gene in B. rapa, relative to its biological clock, allow this plant to grow in different environments with varying temperatures and day lengths.
The clock-regulated genes identified in Brassica are required for primary and secondary metabolism, photosynthesis, cold stress, and response to biotic stimulus [24][19][26][27], which strongly evidences the role of the circadian clock in vegetative growth and plant physiological processes. In line with that, recent works are starting to show that the circadian core clock genes in Brassica crops underlie QTLs that have been related to beneficial influences on key agricultural traits, especially flowering time but also yield, biomass, and growth [28][29][30][31]. In addition, new insights into the circadian clock regulation of other fundamental plant processes, such as responses to abiotic and biotic stresses, could help to guide future work in targeting genes to improve crop growth and stress resilience.
2. Clock-Regulated Phytochemicals
Phytochemicals are specialized compounds that play critical roles in plant adaptation under stressful environmental events [32][33]. Recent advances in the plant metabolome have given unambiguous proof supporting the association of the circadian clock with the daily accumulation of a broad range of plant defense metabolites [34]. Once entrained to cyclic environmental conditions, the accumulation of some phytochemicals, including GSLs and phenolic compounds, shows a rhythmic pattern in the absence of light, temperature, or humidity cues, indicating that this pattern of accumulation is under the control of the circadian clock [35][36]. The variation in phytochemicals in Brassica crops may affect shelf life, food safety, and health benefits that determine the value and drive the consumer perception of quality [37].
2.1. Cyclic Accumulation of Phytochemicals and Nutritional Value
Among all the phytochemicals presents in Brassica vegetables, GSL are the most studied compounds in terms of variation under the control of circadian clock inputs. Rosa et al. [38] showed that GSLs in the leaves of young B. oleracea plants grown under field conditions can vary within a single day. A later study demonstrated that under a constant photoperiod, temperature, light, and relative humidity, individual and total GSLs varied within a 24 h period in kale and cabbage (B. oleracea). This variation was more evident when the temperature was optimum for growth and development [39][40]. It has been shown that, in general, the lowest total GSL levels were observed during the first half of the light cycle with a single recovery in the next hours and reached maximum levels during the dark period. Further research carried out [41] with broccoli (B. oleracea) under semi-field conditions demonstrated that circadian clock rhythms influenced GSL, flavonols, and, in consequence, the sensory quality of broccoli.
More recently, Soengas et al. [35] investigated for species-specific patterns in circadian rhythmicity of GSLs under controlled environmental conditions. Plants from different GSL-containing cultivars belonging to three Brassica species (B. oleracea, B. rapa, and B. napus) were entrained to light–dark cycles prior to release into continuous light. Then, the GSL levels were monitored at four time points of the day during two consecutive days. These experiments corroborated the circadian rhythmicity of total and individual aliphatic GSL levels. However, each cultivar showed a different phase output of maximal GSL accumulation in a day. Even cultivars with a similar aliphatic GSL profile and/or belonging to the same species differ in their shape of GSL fluctuation through time. This suggests that differences could be more related with the plant internal clock rather than the GSL pathway by itself.
Diurnal changes in antioxidant phytochemicals were also observed in Brassica plants. Antioxidant capacity measured using ABTS and DPPH assays found that broccoli heads harvested at the end of the day had higher antioxidants and phenolic compounds than samples harvested in the morning and evening [42]. Soengas et al. [36] reported that the antioxidant activity (measured as ABTS and FRAP) of extracts from different Brassica cultivars belonging to B. oleracea and B. rapa fluctuates rhythmically though the day. In this work, plants were entrained to light–dark cycles prior to release into continuous light. The antioxidant activity and phenolic content was monitored at four time points of the day during four consecutive days (two days under light–dark conditions followed by two days under continuous light). Variations under constant light conditions were related with endogenous circadian rhythms. In general, all the analyzed cultivars accumulated higher levels of individual phenolics correlated with the antioxidant properties at the end of the night period and/or at the beginning of the light period. Since the principal role of plant polyphenols is protection against excess light damages these results evidenced that that Brassica plants anticipate the dawn and adjust their biology accordingly.
2.2. Post-harvest Life of Brassica Vegetables and Circadian Clock
Traditionally, research focused on Brassica food products to delay deterioration during storage has focused on the application of diverse post-harvest treatments such as refrigeration storage, heat treatments, controlled and modified atmospheres, and UV-C light, among others [43]. However, recent advances are starting to show that circadian rhythms [44] and the time of day at which vegetables are harvested [45] could also influence thire post-harvest life. In addition to the discussed daily fluctuations in GSLs and phenolics, changes can be also detected in the metabolism of chlorophyll precursors, sugar metabolism, and ascorbic acid, affecting several aspects of post-harvest performance, which will be discussed in this section.
Liu et al. [44] found that keeping the rhythms of light-dark cycles during post-harvest storage preserved the tissue integrity and nutritional content of leafy plants including Brassica vegetables. The tissue integrity, green coloration, and chlorophyll content from kale and cabbage were generally enhanced by the cycling of light and darkness compared to constant light or darkness during storage. These results were comparable to storage under refrigeration. Similarly, a reduction in senescence was noted for post-harvest broccoli stored under natural light–dark cycles [46]. Therefore, the cycling of light treatment with darkness periods may not only maintain clock function but may also provide the additional benefit of keeping the rhythms of biological processes, such as photosynthesis, during post-harvest storage avoiding physiological damage.
Casajus et al. [45] studied the effect of harvesting at different times of the day on the post-harvest senescence of kale leaves stored at 20 °C in darkness for nine days. They found that leaves harvested in the morning presented earlier symptoms of yellowing, higher rates of chlorophyll degradation, and a lower sugar content compared with leaves harvested at evening. Thus, harvesting in the evening contributes to delaying kale leaves yellowing and senescence metabolism during post-harvest storage. These changes in shelf life are attributed to diurnal changes in leaf status. However, much research is needed to determine whether this effect on post-harvest quality is due to endogenous core clock or is a mere consequence of light.
The effect of light intensity and/or light spectral quality on shelf life, quality, and phytochemical content in post-harvest Brassica vegetables has been investigated [46][47][48][49][50][51]. In this regard, low-intensity light treatment during storage improves the appearance and quality of kale, broccoli, and pak choi by maintaining the chlorophyll content and activating photosynthesis [46][47][51]. In the same way, antioxidants, monosaccharides, and starch were retained in Chinese kale leaves after a treatment with low-intensity continuous light during post-harvest storage [48]. Exposure to white–blue light delayed the senescence of harvested broccoli [49], while the content of total phenols and GSLs was markedly increased by green light [50]. These findings indicate that detached plant organs and post-harvest vegetables maintain responsiveness to light after harvest and more interestingly, if the light intensity is adequate, leaves could continue light-dependent biological process, including photosynthesis and phytochemical retention.
3. The Clock Role on Plant Resistance to Biotic Stress
In the last decade, given the role of biological clocks as master integrators of external information, several authors have focused on the study of the circadian system as a key regulator of plant defense responses against biotic stress. Supporting this notion, several genetic studies demonstrated that Arabidopsis clock mutants show impaired defense responses [52][53][54]. In addition, daily oscillations in susceptibility correlate with circadian regulation of expression of many defense-related genes, including those related with plant secondary metabolites [55][54][56][57]. However, there are only a handful of studies addressing the role of the circadian clock in biotic stress responses. In this section, we will discuss the results obtained in different studies performed with diverse pathogens and insects to elucidate the clock role in plant defense response to biotic threats.
3.1. Pathogen Infection and the Clock
Plant defense mechanisms against pathogens have been reported to vary daily in Arabidopsis. Plants inoculated with bacteria (Pseudomonas syringae) at dusk displayed higher bacterial growth after infection than plants inoculated at dawn did [58]. However, the authors attributed this to a direct effect of light rather than endogenous circadian effects on the induction of plant defense responses. Similarly, the inoculation of Arabidopsis with oomycete (Hyaloperonospora arabidopsidis) at dawn and dusk resulted in significantly higher levels of susceptibility, as measured by sporangiophore counts, at dusk [59]. Nevertheless, the bacterial and sporangiophore counts, which represent the outcome of the plant–pathogen interaction, were not determined under constant conditions in these experiments to confirm endogenous circadian clock regulation of plant defenses. Further work carried out under constant light conditions revealed that the central core clock components CCA1 and LHY act as a positive integrator between the clock and defense pathways in resistance against oomycete and bacterial pathogen infections in Arabidopsis [52][53]. These studies also found that CCA1 and LHY act independently of SA mediated defense but through downstream target genes, such as regulating stomata pathways.
It is well known that stomatal opening is influenced by light intensity, the concentration of atmospheric CO2, endogenous plant hormonal stimuli control, membrane ion transport, and the metabolic activity of guard cells. In addition, current data suggest a link between the circadian system and stomatal opening [60]. In consequence, circadian sensitivity of stomatal responses to pathogens is likely to vary over a day. In the field, the bacterial and sporangiophore counts are higher during the day when stomata are open. This could partially explain why plants accumulate the highest basal defenses during the light period compared with night.
Results from Ingle et al. [57] showed that plant responses to necrotrophic fungus (Botrytis cinerea) are also temporally regulated and play a key role in determining the outcome of infection in Arabidopsis. They profiled the transcriptome of Arabidopsis leaves inoculated with B. cinerea at different times of day and identified a subset of transcription factors that are known to be direct targets of clock proteins that respond differentially to this pathogen depending on the time at which inoculation occurs. Furthermore, they found that jasmonic acid (JA) and ethylene (ET) signaling pathways are involved in this response.
Given the effect of the phytohormones SA, JA, and ET on the contents of GSLs; individual and total phenolic compounds; and carotenoid, chlorophyll and, anthocyanin content by affecting their gene regulation of metabolic biosynthesis, it would be expected that phytochemical accumulation is also involved in the response of the plant clock under pathogen infection. Supporting this notion, a recent study seems to indicate the importance of diurnal rhythms in the accumulation of sulfur-containing defense compounds in B. napus, including GSLs, against the fungal pathogen Verticillium longisporum [61]. Therefore, time of the day and circadian rhythms seem to play a role in the functionality of the plant immune system, which may affect the virulence of pathogens and the overall outcome of host–pathogen relationship. Further work is needed to corroborate this hypothesis.
3.2. Pest Attack and the Clock
The hypothesized interactions between circadian plant defense regulation and pest attack states that tissue defense should be correlated with the probably of biotic stress [62]. Goodspeed et al. [56] carried out the first work reporting advances on the plant clock and defense against herbivory. They show that Arabidopsis resistance against a generalist herbivore insect, Trichoplusia ni, is highly dependent on clock-regulated hormone accumulation. The feeding of T. ni is rhythmic and occurs predominantly around midday. This was consistent with the diurnal accumulation of endogenous JA. Entrainment of circadian rhythms to opposite light–dark cycles in plants and insects results in higher susceptibility of plants to T. ni. In agreement, arrhythmic plants display increased susceptibility to T. ni. To gain insight into this circadian behavior and test whether it can be extended to other plant species, Goodspeed et al. [55] performed a complementary experiment studying the herbivore behavior and plant defense in post-harvest crops. They reported for the first time that post-harvest Brassica crops retain their capacity to entrain diurnal cycles and to enhance their defense. Accordingly, they found that GSLs accumulation of the post-harvest cabbage is dependent on circadian periodicity. These observations suggest that both circadian clock regulation and cyclic activation of defensive compounds are required for a phase-dependent resistance. In addition, mutant analysis showed that the presence of a functional clock enables plants to anticipate upcoming challenges in consonance with pest behavior.
Recently, Lei et al. [54] investigated the link between the circadian clock and plant defense against green peach aphids (Myzus persicae S.). They found that, under constant light, wild-type Columbia-0 (Col-0) Arabidopsis plants entrained with aphids in the same light/dark cycles exhibited a greater antixenotic activity than plants entrained in the opposite cycle from the aphids. Consistently, several loss-of-function clock mutants were more susceptible to aphid infestation than Col-0. However, the arrhythmic CCA1 overexpression line exhibited enhanced resistance toward aphids. The constitutive activation of CCA1 seemed to positively regulate the biosynthesis of indole GSLs. Thus, rhythmic GSLs accumulation has been shown to play a critical role in this time-dependent pest resistance.
Despite the clear links between the clock and immune responses, whether the circadian clock of cultivated Brassica plants affects biotic stress responses remains to be determined. Future research should take into account the considerable different herbivore feeding behaviors that are expected to be occurring in natural habitats. An interesting comparison will be conducting similar experiments to those discussed in this section but with other insects, including night-feeding insects. This could extend our knowledge in the area of plant–insect interactions mediated by the circadian clock.
This entry is adapted from the peer-reviewed paper 10.3390/agronomy11040639
References
- McClung, C.R. The Plant Circadian Oscillator. Biology 2019, 8, 14.
- Fogelmark, K.; Troein, C. Rethinking Transcriptional Activation in the Arabidopsis Circadian Clock. PLoS Comput. Biol. 2014, 10, e1003705.
- Nohales, M.A.; Kay, S.A. Molecular mechanisms at the core of the plant circadian oscillator. Nat. Struct. Mol. Biol. 2016, 23, 1061.
- Harmer, S.L.; Kay, S.A. Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell 2005, 17, 1926–1940.
- Rawat, R.; Takahashi, N.; Hsu, P.Y.; Jones, M.A.; Schwartz, J.; Salemi, M.R.; Phinney, B.S.; Harmer, S.L. REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 2011, 7, e1001350.
- Hsu, P.Y.; Devisetty, U.K.; Harmer, S.L. Accurate timekeeping is controlled by a cycling activator in Arabidopsis. eLife 2013, 2, e00473.
- Dai, S.; Wei, X.; Pei, L.; Thompson, R.L.; Liu, Y.; Heard, J.E.; Ruff, T.G.; Beachy, R.N. BROTHER OF LUX ARRHYTHMO is a component of the Arabidopsis circadian clock. Plant Cell 2011, 23, 961–972.
- Jiang, Y.; Yang, C.; Huang, S.; Xie, F.; Xu, Y.; Liu, C.; Li, L. The ELF3-PIF7 Interaction Mediates the Circadian Gating of the Shade Response in Arabidopsis. iScience 2019, 22, 288–298.
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farré, E.M.; Kay, S.A. The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 2011, 475, 398–402.
- Alabadí, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Más, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883.
- Hazen, S.P.; Schultz, T.F.; Pruneda-Paz, J.L.; Borevitz, J.O.; Ecker, J.R.; Kay, S.A. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc. Natl. Acad. Sci. USA 2005, 102, 10387–10392.
- Farré, E.M.; Harmer, S.L.; Harmon, F.G.; Yanovsky, M.J.; Kay, S.A. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005, 15, 47–54.
- Farré, E.M.; Liu, T. The PRR family of transcriptional regulators reflects the complexity and evolution of plant circadian clocks. Curr. Opin. Plant Biol. 2013, 16, 621–629.
- Helfer, A.; Nusinow, D.A.; Chow, B.Y.; Gehrke, A.R.; Bulyk, M.L.; Kay, S.A. LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Curr. Biol. 2011, 21, 126–133.
- Xu, X.; Xie, Q.; McClung, C.R. Robust Circadian Rhythms of Gene Expression in Brassica rapa Tissue Culture. Plant Physiol. 2010, 153, 841–850.
- Salathia, N.; Lynn, J.R.; Millar, A.J.; King, G.J. Detection and resolution of genetic loci affecting circadian period in Brassica oleracea. Theor. Appl. Genet. 2007, 114, 683–692.
- Lou, P.; Wu, J.; Cheng, F.; Cressman, L.G.; Wang, X.; McClung, C.R. Preferential Retention of Circadian Clock Genes during Diploidization following Whole Genome Triplication in Brassica rapa. Plant Cell 2012, 24, 2415–2426.
- Kim, J.A.; Yang, T.-J.; Kim, J.S.; Park, J.Y.; Kwon, S.-J.; Lim, M.-H.; Jin, M.; Lee, S.C.; Lee, S.I.; Choi, B.-S.; et al. Isolation of circadian-associated genes in Brassica rapa by comparative genomics with Arabidopsis thaliana. Mol. Cells 2007, 23, 145–153.
- Kim, J.A.; Kim, J.S.; Hong, J.K.; Lee, Y.-H.; Choi, B.-S.; Seol, Y.-J.; Jeon, C.H. Comparative mapping, genomic structure, and expression analysis of eight pseudo-response regulator genes in Brassica rapa. Mol. Genet. Genom. 2012, 287, 373–388.
- Song, H.; Yi, H.; Han, C.-T.; Park, J.-I.; Hur, Y. Allelic variation in Brassica oleracea CIRCADIAN CLOCK ASSOCIATED 1 (BoCCA1) is associated with freezing tolerance. Hortic. Environ. Biotechnol. 2018, 59, 423–434.
- Yi, H.; Li, X.; Lee, S.H.; Nou, I.-S.; Lim, Y.P.; Hur, Y. Natural variation in CIRCADIAN CLOCK ASSOCIATED 1 is associated with flowering time in Brassica rapa. Genome 2016, 60, 402–413.
- Xie, Q.; Lou, P.; Hermand, V.; Aman, R.; Park, H.J.; Yun, D.-J.; Kim, W.Y.; Salmela, M.J.; Ewers, B.E.; Weinig, C.; et al. Allelic polymorphism of GIGANTEA is responsible for naturally occurring variation in circadian period in Brassica rapa. Proc. Natl. Acad. Sci. USA 2015, 112, 3829–3834.
- Thiruvengadam, M.; Shih, C.-F.; Yang, C.-H. Expression of An Antisense Brassica oleracea GIGANTEA (BoGI) Gene in Transgenic Broccoli Causes Delayed Flowering, Leaf Senescence, and Post-Harvest Yellowing Retardation. Plant Mol. Biol. Report. 2015, 33, 1499–1509.
- Greenham, K.; Sartor, R.C.; Zorich, S.; Lou, P.; Mockler, T.C.; McClung, C.R. Expansion of the circadian transcriptome in Brassica rapa and genome-wide diversification of paralog expression patterns. eLife 2020, 9, e58993.
- Kim, J.A.; Shim, D.; Kumari, S.; Jung, H.-E.; Jung, K.-H.; Jeong, H.; Kim, W.-Y.; Lee, S.I.; Jeong, M.-J. Transcriptome Analysis of Diurnal Gene Expression in Chinese Cabbage. Genes 2019, 10, 130.
- Xin, H.; Xianchao, N.; Pan, X.; Wei, L.; Min, Y.; Yu, K.; Lunwen, Q.; Wei, H. Comparative Transcriptome Analyses Revealed Conserved and Novel Responses to Cold and Freezing Stress in Brassica napus L. G3 2019, 9, 2723–2737.
- Pu, Y.; Liu, L.; Wu, J.; Zhao, Y.; Bai, J.; Ma, L.; Yue, J.; Jin, J.; Niu, Z.; Fang, Y.; et al. Transcriptome Profile Analysis of Winter Rapeseed (Brassica napus L.) in Response to Freezing Stress, Reveal Potentially Connected Events to Freezing Stress. Int. J. Mol. Sci. 2019, 20, 2771.
- Edwards, C.E.; Ewers, B.E.; Williams, D.G.; Xie, Q.; Lou, P.; Xu, X.; McClung, C.R.; Weinig, C. The genetic architecture of ecophysiological and circadian traits in Brassica rapa. Genetics 2011, 189, 375–390.
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86.
- Jian, H.; Zhang, A.; Ma, J.; Wang, T.; Yang, B.; Shuang, L.S.; Liu, M.; Li, J.; Xu, X.; Paterson, A.H.; et al. Joint QTL mapping and transcriptome sequencing analysis reveal candidate flowering time genes in Brassica napus L. BMC Genom. 2019, 20, 21.
- Akhatar, J.; Goyal, A.; Kaur, N.; Atri, C.; Mittal, M.; Singh, M.P.; Kaur, R.; Rialch, I.; Banga, S.S. Genome wide association analyses to understand genetic basis of flowering and plant height under three levels of nitrogen application in Brassica juncea (L.) Czern & Coss. Sci. Rep. 2021, 11, 4278.
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731.
- Kroymann, J. Natural diversity and adaptation in plant secondary metabolism. Curr. Opin. Plant Biol. 2011, 14, 246–251.
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433.
- Soengas, P.; Cartea, M.E.; Velasco, P.; Francisco, M. Brassica glucosinolate rhythmicity in response to light-dark entrainment cycles is cultivar-dependent. Plant Sci. 2018, 275, 28–35.
- Soengas, P.; Cartea, M.E.; Velasco, P.; Francisco, M. Endogenous Circadian Rhythms in Polyphenolic Composition Induce Changes in Antioxidant Properties in Brassica Cultivars. J. Agric. Food Chem. 2018, 66.
- Francisco, M.; Tortosa, M.; Martínez-Ballesta, M.C.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273–285.
- Rosa, E.A.S.; Heaney, R.K.; Rego, F.C.; Fenwick, G.R. The variation of glucosinolate concentration during a single day in young plants of Brassica oleracea var Acephala and Capitata. J. Sci. Food Agric. 1994, 66, 457–463.
- Rosa, E.A.S.; Rodrigues, P.M.F. The effect of light and temperature on glucosinolate concentration in the leaves and roots of cabbage seedlings. J. Sci. Food Agric. 1998, 78, 208–212.
- Rosa, E.A.S. Daily Variation in Glucosinolate Concentrations in the Leaves and Roots of Cabbage Seedlings in Two Constant Temperature Regimes. J. Sci. Food Agric. 1997, 73, 364–368.
- Mølmann, J.A.B.; Steindal, A.L.H.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem. 2015, 172, 47–55.
- Hasperué, J.H.; Chaves, A.R.; Martínez, G.A. End of day harvest delays postharvest senescence of broccoli florets. Postharvest Biol. Technol. 2011, 59, 64–70.
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147.
- Liu, J.D.; Goodspeed, D.; Sheng, Z.; Li, B.; Yang, Y.; Kliebenstein, D.J.; Braam, J. Keeping the rhythm: Light/dark cycles during postharvest storage preserve the tissue integrity and nutritional content of leafy plants. BMC Plant Biol. 2015, 15, 92.
- Casajús, V.; Perini, M.; Ramos, R.; Lourenco, A.B.; Salinas, C.; Sánchez, E.; Fanello, D.; Civello, P.; Frezza, D.; Martínez, G. Harvesting at the end of the day extends postharvest life of kale (Brassica oleracea var. sabellica). Sci. Hortic. 2021, 276, 109757.
- Büchert, A.M.; Gómez Lobato, M.E.; Villarreal, N.M.; Civello, P.M.; Martínez, G.A. Effect of visible light treatments on postharvest senescence of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2011, 91, 355–361.
- Bárcena, A.; Martínez, G.; Costa, L. Low intensity light treatment improves purple kale (Brassica oleracea var. sabellica) postharvest preservation at room temperature. Heliyon 2019, 5, e02467.
- Noichinda, S.; Bodhipadma, K.; Mahamontri, C.; Narongruk, T.; Ketsa, S. Light during storage prevents loss of ascorbic acid, and increases glucose and fructose levels in Chinese kale (Brassica oleracea var. alboglabra). Postharvest Biol. Technol. 2007, 44, 312–315.
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous white–blue LED light exposition delays postharvest senescence of broccoli. LWT Food Sci. Technol. 2016, 65, 495–502.
- Jin, P.; Yao, D.; Xu, F.; Wang, H.; Zheng, Y. Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chem. 2015, 172, 705–709.
- Yan, Z.; Zuo, J.; Zhou, F.; Shi, J.; Xu, D.; Hu, W.; Jiang, A.; Liu, Y.; Wang, Q. Integrated Analysis of Transcriptomic and Metabolomic Data Reveals the Mechanism by Which LED Light Irradiation Extends the Postharvest Quality of Pak-choi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Biomolecules 2020, 10, 252.
- Bhardwaj, V.; Meier, S.; Petersen, L.N.; Ingle, R.A.; Roden, L.C. Defence Responses of Arabidopsis thaliana to Infection by Pseudomonas syringae Are Regulated by the Circadian Clock. PLoS ONE 2011, 6, e26968.
- Zhang, C.; Xie, Q.; Anderson, R.G.; Ng, G.; Seitz, N.C.; Peterson, T.; McClung, C.R.; McDowell, J.M.; Kong, D.; Kwak, J.M.; et al. Crosstalk between the Circadian Clock and Innate Immunity in Arabidopsis. PLoS Patohog. 2013, 9, e1003370.
- Lei, J.; Jayaprakasha, G.K.; Singh, J.; Uckoo, R.; Borrego, E.J.; Finlayson, S.; Kolomiets, M.; Patil, B.S.; Braam, J.; Zhu-Salzman, K. CIRCADIAN CLOCK-ASSOCIATED1 Controls Resistance to Aphids by Altering Indole Glucosinolate Production. Plant Physiol. 2019, 181, 1344.
- Goodspeed, D.; Liu, J.D.; Chehab, E.W.; Sheng, Z.; Francisco, M.; Kliebenstein, D.J.; Braam, J. Postharvest circadian entrainment enhances crop pest resistance and phytochemical cycling. Curr. Biol. 2013, 23, 1235–1241.
- Goodspeed, D.; Chehab, E.W.; Min-Venditti, A.; Braam, J.; Covington, M.F. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 4674–4677.
- Ingle, R.A.; Stoker, C.; Stone, W.; Adams, N.; Smith, R.; Grant, M.; Carré, I.; Roden, L.C.; Denby, K.J. Jasmonate signalling drives time-of-day differences in susceptibility of Arabidopsis to the fungal pathogen Botrytis cinerea. Plant J. 2015, 84, 937–948.
- Griebel, T.; Zeier, J. Light Regulation and Daytime Dependency of Inducible Plant Defenses in Arabidopsis: Phytochrome Signaling Controls Systemic Acquired Resistance Rather than Local Defense. Plant Physiol. 2008, 147, 790–801.
- Wang, W.; Barnaby, J.Y.; Tada, Y.; Li, H.; Tor, M.; Caldelari, D.; Lee, D.; Fu, X.-D.; Dong, X. Timing of plant immune responses by a central circadian regulator. Nature 2011, 470, 110–114.
- Hassidim, M.; Dakhiya, Y.; Turjeman, A.; Hussien, D.; Shor, E.; Anidjar, A.; Goldberg, K.; Green, R.M. CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and the Circadian Control of Stomatal Aperture. Plant Physiol. 2017, 175, 1864–1877.
- Rupp, S.I.; Hornbacher, J.; Horst-Niessen, I.; Schaarschmidt, F.; Riemenschneider, A.; Papenbrock, J. The Diurnal Rhythm of Brassica napus L. Influences Contents of Sulfur-Containing Defense Compounds and Occurrence of Vascular Occlusions during an Infection with Verticillium longisporum. Agronomy 2020, 10, 1227.
- Jander, G. Timely plant defenses protect against caterpillar herbivory. Proc. Natl. Acad. Sci. USA 2012, 109, 4343–4344.
