NAFLD is the world’s most common chronic liver disease, and its increasing prevalence parallels the global rise in diabetes and obesity. It is characterised by fat accumulation in the liver evolving to non-alcoholic steatohepatitis (NASH), an inflammatory subtype that can lead to liver fibrosis and cirrhosis. An unhealthy diet rich in calories, sugars and saturated fats, and low in polyunsaturated fatty acids, fibre, and micronutrients plays a critical role in the development and progression of this disease. Currently, there is no effective pharmacotherapeutic treatment for NAFLD. Treatment is therefore based on lifestyle modifications including changes to diet and exercise, although it is unclear what the most effective form of intervention is.
- NAFLD
- NASH
- nutrition
- diabetes
- metabolic syndrome
- cardiovascular disease
- fructose
- fatty acids
- protein
- Mediterranean diet
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a general term used to cover a continuum of liver disorders that are distinguished by evidence of excessive fat in the liver (hepatic steatosis) on imaging or histology (macrovesicular steatosis in >5% of hepatocytes), and the absence of secondary causes (alcohol consumption, medications, hereditary disorders) [1][2]. NAFLD is the most common cause of chronic liver disease worldwide and represents a major, growing, and often overlooked public health problem [3][4]. Fatty liver diseases are closely linked with a globalised economy and an increasingly homogenous sociocultural westernised lifestyle [4][5]. It is also closely related to other metabolic diseases such as type 2 diabetes mellitus (T2DM), obesity, metabolic syndrome (MetS), and dyslipidaemia. In addition, a subtype of NAFLD called non-alcoholic steatohepatitis (NASH) has a potentially progressive course, leading to liver fibrosis, cirrhosis, and/or hepatocellular carcinoma (HCC)—conditions which may require treatment through liver transplantation [6].
Nutrition is the principal contributory factor affecting NAFLD development. Thus, different dietary components could modify its natural course [5][6][7][8][9][10][11][12][13], which means it is important to comprehensively discuss the role of nutrition and its main components in the natural history of NAFLD. Accordingly, the objective of this manuscript is to provide an overview of the general aspects of NAFLD and to review the effects of specific nutrients and dietary patterns in the development of the disease.
2. Nutrition and NAFLD
Poor nutrition is the leading contributing factor to the development of NAFLD. This means that a healthy diet, combined with weight loss and increased physical activity, forms the most effective therapeutic approach for NAFLD management. In fact, since no standardised or specific medications have yet been approved to treat NAFLD, treatment currently centres around improving patients’ diets and exercise habits.
Dietary intervention (especially the Mediterranean diet) is effective in normalising aminotransferases and lowering intrahepatic fat, whilst exercise improves insulin sensitivity and decreases BMI [14][15]. NAFLD patients should be advised to maintain a low-calorie diet and stop alcohol and tobacco consumption [16]. A daily caloric restriction of 500–1000 kcal is an extremely effective intervention as primary or secondary prevention against NAFLD. A weight-loss reduction of 3% to 5%, meanwhile, is also associated with decreased NAFLD, but a greater reduction in weight (7–10%) is necessary to achieve NAFLD remission and fibrosis regression. The goal of calorie restriction should therefore be to achieve ≥10% overall body weight loss [17], though some authors advise to not exceed 1.6 kg/week weight reduction so as to avoid a worsening of fibrosis and hepatocyte necrosis [18].
The overall goal is to find the best dietary pattern and macronutrient composition to prevent, attenuate, or reverse hepatic steatosis and its progression to steatohepatitis. Diets that can improve IR, oxidative stress, or inflammation are potentially good candidates to treat NAFLD. Below, we summarise what is currently known about the relationship between the core nutritional elements (carbohydrates, fats, and proteins), food groups, and dietary patterns, and the likelihood of NAFLD.
2.1. Carbohydrate Intake and NAFLD
Carbohydrates are classified as simple (fructose, glucose, galactose) and complex (starch). There is evidence suggesting that lower carbohydrate intake (≤40% of daily energy intake) may be beneficial for NAFLD patients [19] and that it is preferable to avoid consumption of carbohydrates with a high glycaemic index [20]. However, most of the conducted studies focus on fructose consumption and its relationship with NAFLD due to its unique hepatic metabolism and because the rise in the consumption of added sugars, particularly fructose, parallels the increasing incidence of NAFLD (Figure 1).
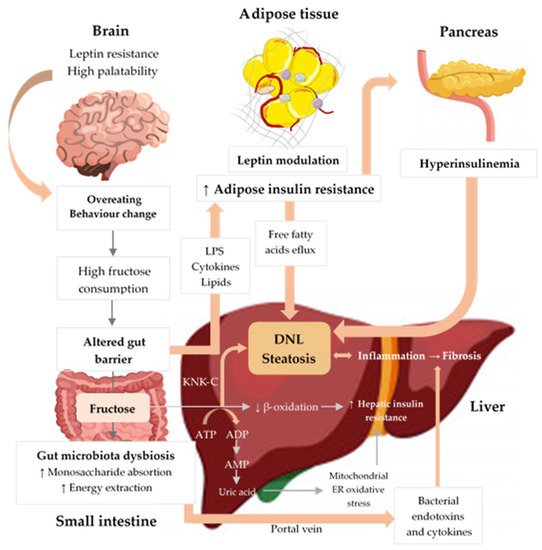
Figure 1. Excessive fructose intake is associated with inflammation, cellular stress, dysbiosis, and hepatic steatosis. Fructose strongly activates DNL, inducing IR and liver inflammation. Fructose metabolism by fructokinase (KHK–C) leads to uric acid generation, mitochondrial dysfunction, and oxidative stress, which contributes to hepatic IR and inflammation. Additionally, high fructose consumption induces gut microbiota dysbiosis, which increases gut permeability, leading to translocation of bacterial endotoxins, cytokines, and lipopolysaccharide (LPS), thus driving hepatic inflammation and IR. Finally, fructose increases peripheral IR through LPS, cytokines, and lipid oxidation and negatively influences appetite through gut–brain axis alterations, high palatability, and leptin modulation, thereby promoting increased energy intake and weight gain [21][22][23][24][25]. Upwards pointing arrows (↑) indicate increase, downwards pointing arrows (↓) indicate decrease.
Fructose is present in natural foods (fruits, vegetables, honey) and in processed foods (juices, nectars, other beverages) [26]. In addition, fructose is a primary component in the most widely used sweeteners (sucrose or high fructose corn syrup [HFCS]). Fructose consumption has increased by 30% in the last 40 years and by 500% over the last century due to the increased consumption of processed foods [27]. Sugar-sweetened beverage (SSB) intake increased by more than 40% from 1990 to 2016 [28]. Today, added sugar intake makes up 15% of total daily calories in the average Western diet [29]. In parallel, there has been a progressively higher incidence and prevalence of obesity, NAFLD, T2DM, and MetS. The increased consumption of added sugars, particularly fructose, is a major underlying cause of chronic metabolic diseases, including NAFLD, T2DM, obesity, hypertension, and CVD [30][31][32][33]. Numerous experimental and clinical studies have found that high fructose consumption is a major risk factor for NAFLD and its consequences [34][35].
Observational studies clearly reveal a close relationship between overconsumption of added sugars and the development of NAFLD in adults and children. A systematic review and meta-analysis of seven studies (six cross-sectional studies and one cohort study) involving 4639 subjects demonstrated that SSB consumers had a 53% increased risk of developing NAFLD compared with non-consumers [35]. Another systematic review and meta-analysis of 12 studies involving 35,705 participants showed that higher consumption of SSBs was positively associated with a 40% increase of NAFLD [11]. Notably, this study found that consumption of SSBs has a dose-dependent effect on the risk of NAFLD. Specifically, low doses (<1 cup/week), medium doses (1–6 cups/week), and high doses (≥7 cups/week) of SSBs significantly increased the relative risk of NAFLD by 14%, 26%, and 53%, respectively. This result was mainly drawn from a cross-sectional study involving 26,790 Chinese adults and showed how a small amount of soft-drink intake is associated with a 14–16% increase in the prevalence of NAFLD, even when adjusted for the presence of MetS [36]. The main limitation of these studies was that they only reflected NAFLD trends in China since almost all data was obtained from the Chinese population. However, other studies with different populations, such as the Framingham Heart Study (n = 2600), also showed a dose–response association [37].
Lastly, a more recent systematic review and meta-analysis (n = 9887) that analysed the influence of all foods on NAFLD development found that dietary intake of added fructose specifically (in the form of sucrose or HFCS) was positively correlated with the likelihood of NAFLD (OR 1.29, CI 95% 0.19–1.40) [38]. It is not yet known if there is a safety threshold for SSB or sugary food consumption for NAFLD prevention [39].
Interventional trials also suggest a role for fructose in NAFLD. For instance, daily consumption of SSBs for 6 months by overweight subjects (n = 47) resulted in the accumulation of liver fat as proven by magnetic resonance spectroscopy [40]. Even short-term interventions demonstrated that consumption of a high-fructose diet (HFruD) had a significant lipogenic effect on the liver [41]. This role was confirmed by a small study that found (n = 16) administering a high-sugar-hypercaloric diet (>1000 kcal simple carbohydrates/day) to subjects for 3 weeks induced DNL, as measured by the lipogenic index [41].
Conversely, a 9-day period of isocaloric fructose restriction in obese children (n = 41) with habitual high fructose consumption showed reductions in liver fat and DNL when compared to controls fed an isocaloric diet [42]. Furthermore, in a subgroup of the same sample, there was an improvement in metabolic parameters such as diastolic blood pressure, serum triglycerides, and IR [43]. While there is consistent evidence for fructose’s causative role in liver fat deposition in a hypercaloric setting, there is conflicting evidence about the impact of carbohydrates on NAFLD in the context of hypocaloric or isocaloric diets. A systematic review and meta-analysis of six observational studies and 21 intervention studies concluded that there was insufficiently robust evidence available to demonstrate that high intake of fructose, HFCS, or sucrose is associated with a higher incidence of NAFLD and suggested that the relationship between fructose intake and NAFLD could be confounded by excessive energy intake [44]. In accordance with this, a recent short-term interventional study found that a high-fructose diet (150 g a day for 8 weeks) in an isocaloric context does not have negative health impacts on weight, liver health, cholesterol, insulin sensitivity, or glucose/blood sugar tolerance in healthy individuals [45]. In this trial with healthy individuals, no change in intramuscular or intrahepatic fat was observed, but there were higher levels of serum triglycerides and DNL. Additionally, a randomised trial that compared a hypocaloric low-carbohydrate diet with a classical hypocaloric low-fat diet showed comparable beneficial results from both interventions; this suggests that total energy deficit is the mediating factor for decreasing liver fat and that carbohydrate consumption has little impact [46].
However, these studies have some limitations [44][45][47]. The follow-up period in these trials is short (mostly 4 weeks or less), whereas we know from animal models that fatty liver develops after at least 8–24 weeks on a high-fructose diet [48]. Other important limitations are the small sample size, poor quality, heterogeneity of the studies, and the fact that most participants were healthy men, which limits the ability to apply the implications of these findings to other populations at high risk for developing NAFLD (T2DM and obese patients).
It is noteworthy that one cross-sectional study from Finland found an inverse relationship between fructose intake and NAFLD. This is because the population studied obtained fructose mostly through fruits and not through sugary drinks [49]. Although fruits contain considerable amounts of simple sugars such as fructose, they are unlikely to induce NAFLD and related diseases for various reasons [50]. Fruits have a lower fructose content per gram (compared to soft drinks) and contain beneficial phytochemicals, micronutrients, and fibre (which is a contributor to overall metabolic health).
In summary, the scientific evidence available to date suggests that chronic, excessive fructose intake is likely a major contributor to NAFLD pathogenesis, especially in genetically predisposed subjects and in the context of hypercaloric diets. Therefore, doctors should encourage their patients with (or at high risk for) NAFLD to reduce added sugar intake, especially fructose.
2.2. Fat Intake and NAFLD
Dietary intervention studies performed in animal and human models have analysed the effect of fats on NAFLD [51]. Most studies suggest that high fat consumption plays a role in NAFLD’s pathogenesis [52][53]. However, monounsaturated, polyunsaturated, and saturated fatty acids (MUFAs, PUFAs, SFAs, respectively) or trans fatty acids (TFAs) do not exert the same effect on the liver, and the main source of each of them is different.
Interventional studies performed in mice show that a long-term high-fat diet promotes NAFLD development [51][52][53][54][55]. For example, an 80-week high-fat diet (60% fat, 20% protein, and 20% carbohydrate) in mice led to obesity and IR, while histological analysis demonstrated liver steatosis, cell injury, inflammation, and fibrosis [54].
Epidemiological studies show that NAFLD patients typically have greater intakes of SFAs and cholesterol and a lower PUFA intake, compared to matched controls without NAFLD [56][57]. The findings of some experimental studies with human beings confirm these results. In an 8-week double-blind randomised trial with obese individuals (n = 61), SFAs increased liver fat content (50% relative increase) and serum ceramides, whereas PUFAs did not [58]. Additionally, a 10-week randomised trial (n = 67) comparing two isocaloric diets containing Omega-6 PUFAs or SFAs showed that Omega-6 PUFAs supplementation caused a reduction in hepatic steatosis, insulin levels, and inflammatory markers when compared with SFAs, though no changes in weight were observed [59]. In another small clinical trial, 38 overweight subjects were overfed for 3 weeks with different macronutrients (unsaturated fats, SFAs, and simple carbohydrates) and showed intrahepatic triglyceride (IHTG) increases of 55%, 33%, and 15% in the SFA, carbohydrate, and unsaturated fat groups, respectively. Furthermore, SFAs induced IR and endotoxemia and significantly increased multiple plasma ceramides [60]. This effect was also confirmed in healthy adults taking a palm oil bolus, which resulted in a measurable increase in IHTG, energy metabolism, and IR [61].
In general, NAFLD patients consume less Omega-3 PUFAs and a higher Omega-6:Omega-3 ratio, compared to healthy controls [9]. Dietary Omega-3 PUFAs are mainly found in fish, seeds, walnuts, and some plant oils. A cross-sectional study in the paediatric population (n = 223) found that most children with NAFLD have insufficient intake of Omega-3 PUFAs (<200 mg/day) [62]. Indeed, as precursors of eicosanoids, Omega-3 fatty acids have an anti-inflammatory effect, regulate hepatic lipid composition, and improve IR [63][64], whereas Omega-6 fatty acids are proinflammatory and could have a negative effect on NAFLD [65].
In fact, many systematic reviews and meta-analyses of randomised controlled trials have addressed the topic of Omega-3 supplementation and its effect on patients suffering from NAFLD. These studies conclude that Omega 3 PUFAs supplementation (>3 g/day) is useful for the reduction of liver fat, hepatic enzymes, BMI, triglycerides, and cholesterol [66][67]. This potentially sets Omega-3 food supplementation as a safe, viable, and effective intervention to help treat NAFLD.
Regarding the role of MUFA intake in the prevention of NAFLD, there are heterogeneous results: observational studies suggest a neutral effect [68][69], while interventional studies such as the PREDIMED study show a beneficial effect with extra virgin olive oil (EVOO) supplementation (90% of fat in EVOO is MUFA) [70]. Some small, short-term randomised clinical trials have also shown clear positive results [71][72]. Thus, the consumption of a high-MUFA diet (28% of total caloric intake) for 8 weeks by T2DM patients reduced liver fat by 29% without body weight changes, in comparison to a baseline diet moderately rich in SFAs (13% of total energy). Recently, a double-blind randomised controlled clinical trial with NAFLD patients (n = 66) found that consumption of 20 g/day of olive oil attenuated fatty liver grade and reduced body fat percentage [73]. It has been reported that MUFA may prevent the development of NAFLD by improving plasma lipid levels, reducing body fat accumulation, and decreasing postprandial adiponectin expression [74]. Based on these results, the EASL-EAS-EASO Clinical Guidelines recommend the Mediterranean diet for NAFLD subjects due to its high content of MUFAs [75].
Finally, TFA consumption has a pro-oxidative effect and is associated with an increased risk for CVD, IR, obesity, and systemic inflammation and may also damage the liver [76]. These data are mainly based on population studies such as the Rotterdam study (n = 3882), which indicates that TFAs found in desserts and processed foods were associated with a higher prevalence of NAFLD [69]. Due to its unhealthy effects, there is no human clinical trial to assess the effect of TFA intake in the liver.
In summary, the effect of fat intake on NAFLD development depends on the type of fat. Some fats (MUFA and PUFA) protect against NAFLD whereas others (SFA and TFA) have a negative effect on NAFLD. However, most of the studies are observational, and only a limited number of small clinical trials have been published to date. Although the evidence is of low-moderate quality, and there are still many questions to be resolved, clinicians should nevertheless advise their patients to lower SFA intake, eliminate TFAs, and increase Omega-3 PUFA to reduce the incidence of NAFLD.
2.3. Protein Intake and NAFLD
Little is known about the influence of protein and amino acids on NAFLD since most of the intervention and mechanistic studies have focused on carbohydrates and fats. Furthermore, intervention and observational studies on the relationship between protein and NAFLD show conflicting results. Some studies found evidence that higher protein intakes might have a negative effect on NAFLD, while other studies showed a neutral or positive effect [77][78]. These inconsistencies might be explained by the type of protein consumed, as animal protein seems to have a negative effect on NAFLD, while plant protein has an inverse association [77]. It is well established that high meat intake, especially of red meats and processed meats, is associated with IR, T2DM, and CVD [79][80][81]. NAFLD has also been associated with red and processed meat consumption even when intakes are low [82][83]. However, this effect may be because of the saturated fat content, salt, additives, and cooking methods, more than the effect of the protein itself.
In conclusion, the effects of protein on the development of NAFLD remain unclear, but they seem positive, especially with vegetable origin proteins, which is likely due to their high fibre and phytonutrient content. The specific problems associated with animal proteins may be related to the fact that they are usually consumed jointly with SFAs (meat products).
2.4. Fibre Intake and NAFLD
The relationship between fibre intake and NAFLD has been assessed in several observational studies and in a small number of small clinical trials with conflicting results. Although most of the studies suggest that high daily fibre consumption is associated with a preventive effect against NAFLD, other authors have not confirmed these results [84]. Recently, a study performed in a Dutch population found that subjects with a high fatty liver index (FLI) consumed less fibre daily, compared to those with a low FLI [68]. Similar results were also obtained in a subgroup of 6613 US adults participating in the National Health and Nutrition Examination Survey [84]. However, it is not fully known whether the protective effect of fibre against NAFLD is due to an indirect action through modulation of the microbiome or if it is a result of the direct anti-inflammatory properties of fibre [26].
2.5. Food Groups
Several epidemiological studies have attempted to assess the relationship between different food groups and the risk of NAFLD; however, the results obtained were quite heterogeneous. Recently, Chinese investigators performed a meta-analysis to study the association between eleven food groups (red meat, soft drinks, nuts, whole grains, refined grains, fish, fruits, vegetables, eggs, dairy products, and legumes) and the probability of developing NAFLD. They collected 24 studies (15 cross-sectional and 9 case–control studies) and made the following conclusions: (1) there is a positive association between red meat and soft drink consumption and the risk of NAFLD, (2) there is a negative association between nut consumption and the likelihood of NAFLD, and (3) no significant causal relationship was observed between the other food groups and NAFLD. Although this study had some limitations (reduced number of studies, risk of bias could not be tested, most studies did not stratify food intake), the results obtained were consistent with the current recommended guidelines for treating NAFLD [75][38].
2.6. Dietary Patterns and NAFLD
2.6.1. Low and Very Low Carbohydrate Diets (Ketogenic Diet)
Low/very low carbohydrate diets are dietary patterns that restrict the overall intake of carbohydrates. In the ketogenic diet (KD), carbohydrate content is kept below 10% of total daily caloric intake (20–50 g of carbohydrate/day) [85], although the specific macronutrient composition may vary. The KD is a powerful tool to induce weight loss and achieve greater long-term weight reduction, compared to low-fat diets [86].
With respect to NAFLD, the effect of low-carbohydrate diets remains extremely controversial [18]. Several small clinical trials have confirmed that obese patients following a KD improve liver steatosis, inflammation, IR, and dyslipidaemia and achieve a significant reduction in body weight in the short term [87][88]. Nevertheless, some authors suggest that these effects are lost in the long term (>12 months) [89]. However, the results obtained with a KD are quite heterogeneous, and the samples are very small; thus, it is difficult to draw conclusions that can be applied to the general population. In addition, it is not possible to determine whether the beneficial effects are due to weight loss, calorie restriction, or changes in macronutrient distribution. Some authors suggest that the KD could be a practical short-term approach to NAFLD because it may offer an opportunity to exclude sugary foods from the diet and because the greater weight reduction associated with a KD makes it easier to achieve a normal BMI [90].
2.6.2. Mediterranean Diet
The Mediterranean diet (MedDiet) is composed in the following way: it is typically rich in vegetables, fruits, whole grains, legumes, nuts, and seeds, and EVOO; it is moderate in fish and other meats, dairy products, and red wine; and it involves a low intake of eggs, red meat, and sweets. As a result, it is a diet rich in antioxidants and fibre, with little saturated fat or animal protein, and with an appropriate Omega-3:Omega-6 fatty acid ratio [91]. It has several health benefits, including a lower incidence of CVD and components of MetS [19][92][93]. In addition, several studies (observational studies and short-term trials) have demonstrated that the MedDiet is beneficial due to its effect on most risk factors for NAFLD (body weight, serum triglycerides, and IR) [94][95]. A recent short-term, small clinical trial (n = 49) showed that an ad libitum low-fat diet and MedDiet reduced hepatic steatosis by approximately 25% and was associated with minimal weight loss (−2.1 kg) [96]. Another clinical trial with 63 overweight or obese participants observed that the combination of a MedDiet and increased physical activity had a greater effect against NAFLD than a MedDiet alone [97].
Some authors suggest that the use of the Mediterranean adequacy index—which is obtained from the ratio of the combined percentage of total energy from Mediterranean foods and the total energy from non-Mediterranean foods—is a useful tool to assess adherence to the MedDiet. A daily score greater than 5 is necessary to obtain a health benefit [22]. Today, a growing body of evidence suggests that the MedDiet, in combination with exercise and behaviour therapy, may be the reference nutritional pattern to prevent and treat NAFLD [95]. In fact, most guidelines recommend the MedDiet as the best dietary pattern against liver steatosis [98][99]. However, it is necessary to perform more clinical trials with larger sample sizes in order to obtain the best scientific evidence on this issue, standardise the MedDiet characteristics, and develop specific tools to assess adherence to this type of diet.
2.6.3. Vegetarian and Vegan Diets
These diets are based on low or no consumption of animal products and a high intake of vegetables, legumes, fruits, whole grains, and fibre, which are all rich in polyphenols with antioxidant and anti-inflammatory properties. Many studies have noted that plant-based diets have a protective effect against multiple diseases related to NAFLD such as T2DM, MetS, hypertension, obesity, CVD, and all-cause mortality [100][101][102][103]. Furthermore, several randomised controlled studies demonstrated that a vegan diet could be more efficient for weight loss than other eating patterns including a vegetarian diet or a MedDiet [104][105]. Population studies such as NHANES and the Rotterdam study indicated that high adherence to a plant-based diet was associated with improvement in risk factors (IR, BMI) related to NAFLD development [106][107]. A cross-sectional study (n=3279) analysed food substitution in vegetarians and found that meat eaters had a 12% greater risk of developing NAFLD than vegetarians, suggesting that replacing animal protein with plant-based protein may prevent fatty liver [108]. Although these dietary patterns involve higher fructose intake, they do not seem to be associated with major incidences of NAFLD. This paradoxical situation may be due to the fact that the main source of fructose is whole fruit [109]. In summary, there is a lack of randomised controlled clinical trials assessing the effect of a vegan/vegetarian diet against NAFLD. Although the evidence is of low quality, it seems that plant-based diets can exert a protective effect against NAFLD.
2.6.4. Dietary Approaches to Stop Hypertension (DASH Diet)
DASH is a sodium-restricted diet (<2400 mg/day) that is rich in fruits, vegetables, whole grains, low-fat dairy products, and lean protein; it is similar to the MedDiet but has a special focus on reducing total sodium intake [110]. Evidence shows that the DASH diet improves some risk factors for NAFLD (T2DM, MetS, obesity, dyslipidaemia). Furthermore, a systematic review and meta-analysis reported that DASH might be a better choice for weight management and reduction than other low-energy diets [111]. Some observational studies suggest that this diet could play a preventive role in NAFLD [112][113].
In fact, small short-term clinical trials have observed that NAFLD patients who adhere to a DASH diet achieve a reduction in body weight/BMI and improve triglyceride, ALT, AST, insulin sensitivity, and inflammatory marker serum levels [114]. Unfortunately, however, compliance with the diet remains low [115]. Therefore, it is thought that the DASH diet can be a useful tool to tackle NAFLD, but comparative clinical trials are still needed to assess the real effects of this diet on specific markers of NAFLD (histological and metabolic parameters).
2.6.5. Intermittent Fasting
Intermittent fasting (IF) is an emerging nutritional approach for weight loss and for the management of metabolic diseases and is considered an alternative to continuous calorie restriction [116]. The basic concept of IF involves regularly alternating periods of eating and fasting with either total food abstinence or very low energy intake [117]. IF has many forms, with the most common being alternate-day fasting (ADF), time-restricted feeding (TRF), or prolonged fasting. A recent systematic review and meta-analysis that included interventional trials concluded that IF was comparable to continuous energy restriction for short-term weight loss in individuals with overweight and obesity [118]. Since the primary goal of NAFLD therapy is weight loss, it is plausible that IF positively influences liver steatosis and metabolic markers. Another key mechanism responsible for the beneficial effects of IF (apart from the global caloric restriction) appears to be the body’s preferential shift from glycogenolysis-derived glucose to lipolysis-derived ketones [119]. Therefore, these protocols may have potential as a useful intervention in NAFLD patients. However, there is not yet scientific evidence to confirm these theoretical protective effects of IF against NAFLD.
This entry is adapted from the peer-reviewed paper 10.3390/nu13051442
References
- Rinella, M.E. Nonalcoholic fatty liver disease. JAMA 2015, 313, 2263.
- Kupčová, V.; Fedelešová, M.; Bulas, J.; Kozmonová, P.; Turecký, L. Overview of the Pathogenesis, Genetic, and Non-Invasive Clinical, Biochemical, and Scoring Methods in the Assessment of NAFLD. Int. J. Environ. Res. Public Health 2019, 16, 3570.
- Lazarus, J.V.; Colombo, M.; Cortez-Pinto, H.; Huang, T.T.-K.; Miller, V.; Ninburg, M.; Schattenberg, J.M.; Seim, L.; Wong, V.W.S.; Zelber-Sagi, S. NAFLD—Sounding the alarm on a silent epidemic. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 377–379.
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16.
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904.
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis. JAMA 2020, 323, 1175.
- Chakravarthy, M.V.; Waddell, T.; Banerjee, R.; Guess, N. Nutrition and Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. N. Am. 2020, 49, 63–94.
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108.
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of Nutritional Changes on Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 677.
- George, E.S.; Forsyth, A.; Itsiopoulos, C.; Nicoll, A.J.; Ryan, M.; Sood, S.; Roberts, S.; Tierney, A.C. Practical Dietary Recommendations for the Prevention and Management of Nonalcoholic Fatty Liver Disease in Adults. Adv. Nutr. 2018, 9, 30–40.
- Chen, H.; Wang, J.; Li, Z.; Lam, C.W.K.; Xiao, Y.; Wu, Q.; Zhang, W. Consumption of Sugar-Sweetened Beverages Has a Dose-Dependent Effect on the Risk of Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Dose-Response Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2192.
- Boutari, C.; Lefkos, P.; Athyros, V.G.; Karagiannis, A.; Tziomalos, K. Nonalcoholic Fatty Liver Disease vs. Nonalcoholic Steatohepatitis: Pathological and Clinical Implications. Curr. Vasc. Pharmacol. 2018, 16, 214–218.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Zou, T.-T.; Zhang, C.; Zhou, Y.-F.; Han, Y.-J.; Xiong, J.-J.; Wu, X.-X.; Chen, Y.-P.; Zheng, M.-H. Lifestyle interventions for patients with nonalcoholic fatty liver disease: A network meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 747–755.
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846.
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease. JAMA Intern. Med. 2019, 179, 1262.
- Hydes, T.J.; Ravi, S.; Loomba, R.; Gray, M.E. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 2020, 26, 383–400.
- Asrih, M.; Jornayvaz, F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014, 33, 186–190.
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. NEJM 2018, 378, e34.
- Bawden, S.; Stephenson, M.; Falcone, Y.; Lingaya, M.; Ciampi, E.; Hunter, K.; Bligh, F.; Schirra, J.; Taylor, M.; Morris, P.; et al. Increased liver fat and glycogen stores after consumption of high versus low glycaemic index food: A randomized crossover study. Diabetesobesity Metab. 2016, 19, 70–77.
- Duarte, S.M.B.; Stefano, J.T.; Oliveira, C.P. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH). Ann. Hepatol. 2019, 18, 416–421.
- Jegatheesan, P.; De Bandt, J. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230.
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322.
- Zhang, D.-M.; Jiao, R.-Q.; Kong, L.-D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335.
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555.
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, Genetic Factors, and Their Interaction in Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8761.
- Johnson, R.J.; Sánchez-Lozada, L.G.; Andrews, P.; Lanaspa, M.A. Perspective: A Historical and Scientific Perspective of Sugar and Its Relation with Obesity and Diabetes. Adv. Nutr. 2017, 8, 412–422.
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422.
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682.
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075.
- Rippe, J.M.; Angelopoulos, T.J. Fructose-Containing Sugars and Cardiovascular Disease. Adv. Nutr. 2015, 6, 430–439.
- Tappy, L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018, 221.
- Macdonald, I.A. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur. J. Nutr. 2016, 55, 17–23.
- Schwarz, J.-M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442.
- Wijarnpreecha, K.; Thongprayoon, C.; Edmonds, P.J.; Cheungpasitporn, W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: A systematic review and meta-analysis. QJM 2015, 109, 461–466.
- Meng, G.; Zhang, B.; Yu, F.; Li, C.; Zhang, Q.; Liu, L.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; et al. Soft drinks consumption is associated with nonalcoholic fatty liver disease independent of metabolic syndrome in Chinese population. Eur. J. Nutr. 2017, 57, 2113–2121.
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469.
- He, K.; Li, Y.; Guo, X.; Zhong, L.; Tang, S. Food groups and the likelihood of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Br. J. Nutr. 2020, 124, 1–13.
- European Food Safety Authority (EFSA) Protocol for the scientific opinion on the Tolerable Upper Intake Level of dietary sugars. Efsa J. 2018, 16.
- Sobrecases, H.; Lê, K.-A.; Bortolotti, M.; Schneiter, P.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010, 36, 244–246.
- Sevastianova, K.; Santos, A.; Kotronen, A.; Hakkarainen, A.; Makkonen, J.; Silander, K.; Peltonen, M.; Romeo, S.; Lundbom, J.; Lundbom, N.; et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am. J. Clin. Nutr. 2012, 96, 727–734.
- Schwarz, J.-M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology 2017, 153, 743–752.
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-Cakmak, A.; Gugliucci, A.; Schwarz, J.-M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 2016, 24, 453–460.
- Chung, M.; Ma, J.; Patel, K.; Berger, S.; Lau, J.; Lichtenstein, A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 833–849.
- Smajis, S.; Gajdošík, M.; Pfleger, L.; Traussnigg, S.; Kienbacher, C.; Halilbasic, E.; Ranzenberger-Haider, T.; Stangl, A.; Beiglböck, H.; Wolf, P.; et al. Metabolic effects of a prolonged, very-high-dose dietary fructose challenge in healthy subjects. Am. J. Clin. Nutr. 2019, 111, 369–377.
- Haufe, S.; Engeli, S.; Kast, P.; Böhnke, J.; Utz, W.; Haas, V.; Hermsdorf, M.; Mähler, A.; Wiesner, S.; Birkenfeld, A.L.; et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011, 53, 1504–1514.
- Chiu, S.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423.
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.-K.; Mirshahi, F.; et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 2016, 65, 579–588.
- Kanerva, N.; Sandboge, S.; Kaartinen, N.E.; Männistö, S.; Eriksson, J.G. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am. J. Clin. Nutr. 2014, 100, 1133–1138.
- Sharma, S.; Chung, H.; Kim, H.; Hong, S. Paradoxical Effects of Fruit on Obesity. Nutrients 2016, 8, 633.
- Green, C.; Hodson, L. The Influence of Dietary Fat on Liver Fat Accumulation. Nutrients 2014, 6, 5018–5033.
- Velázquez, K.T.; Enos, R.T.; Bader, J.E.; Sougiannis, A.T.; Carson, M.S.; Chatzistamou, I.; Carson, J.A.; Nagarkatti, P.; Nagarkatti, M.; Murphy, E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019, 11, 619–637.
- Mells, J.E.; Fu, P.P.; Kumar, P.; Smith, T.; Karpen, S.J.; Anania, F.A. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J. Nutr. Biochem. 2015, 26, 285–292.
- Parks, E.; Yki-Järvinen, H.; Hawkins, M. Out of the frying pan: Dietary saturated fat influences nonalcoholic fatty liver disease. J. Clin. Investig. 2017, 127, 454–456.
- Yang, P.; Wang, Y.; Tang, W.; Sun, W.; Ma, Y.; Lin, S.; Jing, J.; Jiang, L.; Shi, H.; Song, Z.; et al. Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice. Sci. Rep. 2020, 10, 4701.
- Fan, J.-G.; Cao, H.-X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2013, 28, 81–87.
- Zelber-Sagi, S. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World Journal of Gastroenterology 2011, 17, 3377.
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.-E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219.
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012.
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739.
- Hernández, E.Á.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708.
- St-Jules, D.E.; Watters, C.A.; Brunt, E.M.; Wilkens, L.R.; Novotny, R.; Belt, P.; Lavine, J.E. Estimation of Fish and ω-3 Fatty Acid Intake in Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatric Gastroenterol. Nutr. 2013, 57, 627–633.
- Eslamparast, T.; Tandon, P.; Raman, M. Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 800.
- de Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55.
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 1–16.
- Lee, C.-H.; Fu, Y.; Yang, S.-J.; Chi, C.-C. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2769.
- Guo, X.; Yang, B.; Tang, J.; Li, D. Fatty acid and non-alcoholic fatty liver disease: Meta-analyses of case-control and randomized controlled trials. Clin. Nutr. 2018, 37, 113–122.
- Rietman, A.; Sluik, D.; Feskens, E.J.M.; Kok, F.J.; Mensink, M. Associations between dietary factors and markers of NAFLD in a general Dutch adult population. Eur. J. Clin. Nutr. 2017, 72, 117–123.
- Alferink, L.J.; Kiefte-de Jong, J.C.; Erler, N.S.; Veldt, B.J.; Schoufour, J.D.; de Knegt, R.J.; Ikram, M.A.; Metselaar, H.J.; Janssen, H.L.; Franco, O.H.; et al. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: The Rotterdam Study. Gut 2018, 68, 1088–1098.
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929.
- Bozzetto, L.; Prinster, A.; Annuzzi, G.; Costagliola, L.; Mangione, A.; Vitelli, A.; Mazzarella, R.; Longobardo, M.; Mancini, M.; Vigorito, C.; et al. Liver Fat Is Reduced by an Isoenergetic MUFA Diet in a Controlled Randomized Study in Type 2 Diabetic Patients. Diabetes Care 2012, 35, 1429–1435.
- Nigam, P.; Bhatt, S.; Misra, A.; Chadha, D.S.; Vaidya, M.; Dasgupta, J.; Pasha, Q.M.A. Effect of a 6-Month Intervention with Cooking Oils Containing a High Concentration of Monounsaturated Fatty Acids (Olive and Canola Oils) Compared with Control Oil in Male Asian Indians with Nonalcoholic Fatty Liver Disease. Diabetes Technol. Ther. 2014, 16, 255–261.
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161.
- Paniagua, J.A.; de la Sacristana, A.G.; Romero, I.; Vidal-Puig, A.; Latre, J.M.; Sanchez, E.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Monounsaturated Fat-Rich Diet Prevents Central Body Fat Distribution and Decreases Postprandial Adiponectin Expression Induced by a Carbohydrate-Rich Diet in Insulin-Resistant Subjects. Diabetes Care 2007, 30, 1717–1723.
- EASL; EASD; EASO. EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402.
- Dhibi, M.; Brahmi, F.; Mnari, A.; Houas, Z.; Chargui, I.; Bchir, L.; Gazzah, N.; Alsaif, M.A.; Hammami, M. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr. Metab. 2011, 8, 65.
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8.
- Lang, S.; Martin, A.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.J.G.T.; Liu, J.; Krawczyk, M.; Nowag, A.; Kretzschmar, A.; Herweg, J.; et al. High Protein Intake Is Associated With Histological Disease Activity in Patients With NAFLD. Hepatol. Commun. 2020, 4, 681–695.
- Turner, K.M.; Keogh, J.B.; Clifton, P.M. Red meat, dairy, and insulin sensitivity: A randomized crossover intervention study. Am. J. Clin. Nutr. 2015, 101, 1173–1179.
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375.
- de Medeiros, G.C.B.S.; de Azevedo, K.P.M.; Mesquita, G.X.B.; Lima, S.C.V.C.; de Oliveira Silva, D.F.; Pimenta, I.D.S.F.; da Silveira Gonçalves, A.K.; de Oliveira Lyra, C.; Piuvezam, G. Red meat consumption, risk of incidence of cardiovascular disease and cardiovascular mortality, and the dose–response effect. Medicine 2019, 98, e17271.
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246.
- Hashemian, M.; Poustchi, H.; Merat, S.; Abnet, C.; Malekzadeh, R.; Etemadi, A. Red Meat Consumption and Risk of Nonalcoholic Fatty Liver Disease in a Population with Low Red Meat Consumption. Curr. Dev. Nutr. 2020, 4, 1413.
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association between Dietary Fiber Intake and Non-alcoholic Fatty Liver Disease in Adults. Front. Nutr. 2020, 7, 269.
- Choi, Y.J.; Jeon, S.-M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005.
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187.
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5.
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354.
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639.
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024.
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942.
- Agnoli, C.; Sieri, S.; Ricceri, F.; Giraudo, M.T.; Masala, G.; Assedi, M.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr. Diabetes 2018, 8, 22.
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222.
- Suárez, M.; Boqué, N.; del Bas, J.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052.
- Akhlaghi, M.; Ghasemi-Nasab, M.; Riasatian, M. Mediterranean diet for patients with non-alcoholic fatty liver disease, a systematic review and meta-analysis of observational and clinical investigations. J. Diabetes Metab. Disord. 2020, 19, 575–584.
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754.
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011.
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.-V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175.
- Glen, J.; Floros, L.; Day, C.; Pryke, R. Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ 2016, 354, i4428.
- Toumpanakis, A.; Turnbull, T.; Alba-Barba, I. Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: A systematic review. BMJ Open Diabetes Res. Care 2018, 6, e000534.
- Turner-McGrievy, G.; Harris, M. Key Elements of Plant-Based Diets Associated with Reduced Risk of Metabolic Syndrome. Curr. Diabetes Rep. 2014, 14, 524.
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian Diets and Blood Pressure. Jama Intern. Med. 2014, 174, 577.
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079.
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A Systematic Review and Meta-Analysis of Changes in Body Weight in Clinical Trials of Vegetarian Diets. J. Acad. Nutr. Diet. 2015, 115, 954–969.
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrition 2015, 31, 350–358.
- Alferink, L.J.M.; Erler, N.S.; de Knegt, R.J.; Janssen, H.L.A.; Metselaar, H.J.; Darwish Murad, S.; Kiefte-de Jong, J.C. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur. J. Epidemiol. 2020, 35, 1069–1085.
- Mazidi, M.; Kengne, A.P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 2018, 38, 1672–1677.
- Lin, C.-L.; Chiu, T.; Lin, M.-N.; Pan, W.-H.; Chen, Y.-C. Vegetarian diet, food substitution, and nonalcoholic fatty liver. Tzu Chi Med. J. 2018, 30, 102.
- Tajima, R.; Kimura, T.; Enomoto, A.; Saito, A.; Kobayashi, S.; Masuda, K.; Iida, K. No association between fruits or vegetables and non-alcoholic fatty liver disease in middle-aged men and women. Nutrition 2019, 61, 119–124.
- Steinberg, D.; Bennett, G.G.; Svetkey, L. The DASH Diet, 20 Years Later. JAMA 2017, 317, 1529.
- Soltani, S.; Shirani, F.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Obes. Rev. 2016, 17, 442–454.
- Park, S.-Y.; Noureddin, M.; Boushey, C.; Wilkens, L.R.; Setiawan, V.W. Diet Quality Association with Nonalcoholic Fatty Liver Disease by Cirrhosis Status: The Multiethnic Cohort. Curr. Dev. Nutr. 2020, 4, nzaa024.
- Watzinger, C.; Nonnenmacher, T.; Grafetstätter, M.; Sowah, S.A.; Ulrich, C.M.; Kauczor, H.-U.; Kaaks, R.; Schübel, R.; Nattenmüller, J.; Kühn, T. Dietary Factors in Relation to Liver Fat Content: A Cross-sectional Study. Nutrients 2020, 12, 825.
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2015, 36, 563–571.
- Kwan, M.W.-M.; Wong, M.C.-S.; Wang, H.H.-X.; Liu, K.Q.-L.; Lee, C.L.-S.; Yan, B.P.-Y.; Yu, C.-M.; Griffiths, S.M. Compliance with the Dietary Approaches to Stop Hypertension (DASH) Diet: A Systematic Review. PLoS ONE 2013, 8, e78412.
- Park, J.; Seo, Y.-G.; Paek, Y.-J.; Song, H.J.; Park, K.H.; Noh, H.-M. Effect of alternate-day fasting on obesity and cardiometabolic risk: A systematic review and meta-analysis. Metabolism 2020, 111, 154336.
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873.
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547.
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268.
