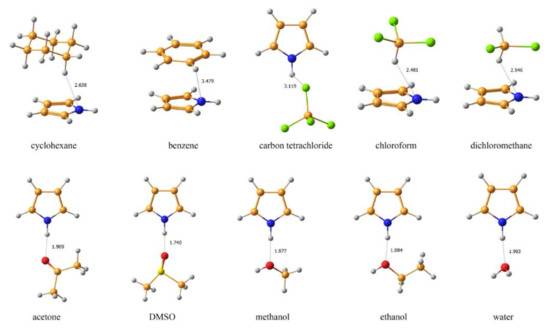
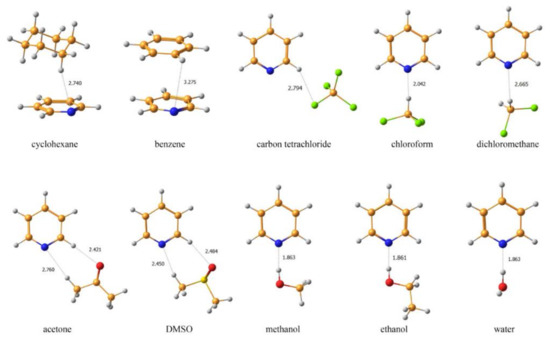
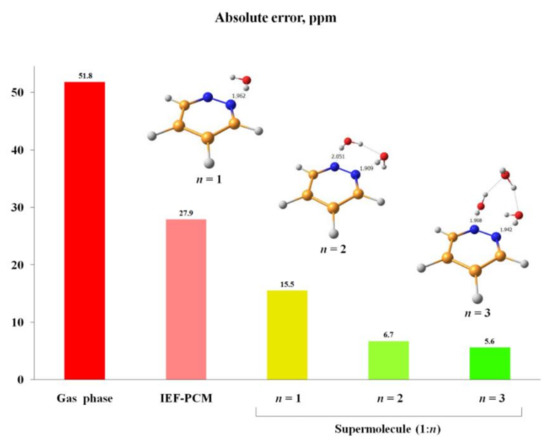
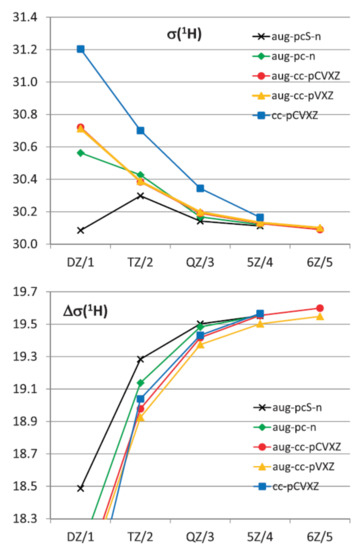
3. Monosaccharides
Two recent comprehensive reviews from Serianni’s group dealing with the computational aspects [
1] and stereochemical applications [
2] of NMR chemical shifts and spin-spin coupling constants of saccharides together with a review by Toukach and Ananikov [
7] have recently appeared. In the present review, we will not go into much detail, only covering in general the most illustrative examples in this field.
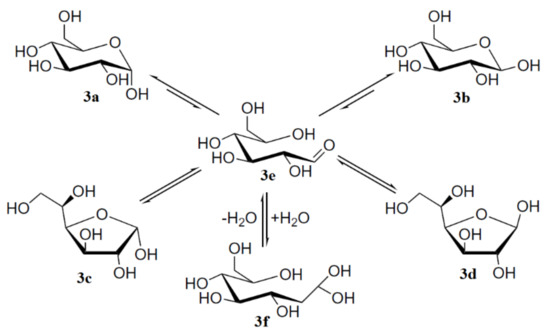
To begin with, it is well known that mutarotation of monosaccharides proceeds via the interconvertion of two hexopyranose and two pentofuranose forms through the open-chain intermediate which is exemplified below by the mutarotation of the most classical monosaccharide D-glucose, which was studied in much detail by Zhu and coauthors [
110]. The results of that study demonstrated that concentrated solutions of aldohexoses in water represented almost all forms of D-glucose: α-pyranose, 37.63% (
3a); β-pyranose, 61.96% (
3b); α-furanose, 0.108% (
3c); β-furanose, 0.28% (
3d); together with the traces of the aldehyde form, 0.0040% (
3e) and the hydrate form, 0.0059% (
3f) shown in
Scheme 1, the latter retrieved from Roslund et al. [
111].
Scheme 1. Mutarotation of D-glucose in solution. Reproduced from Roslund et al. [
111] with the permission of Elsevier.
In the cited study of the mutarotation of D-glucose in solution [
111], the
1H and
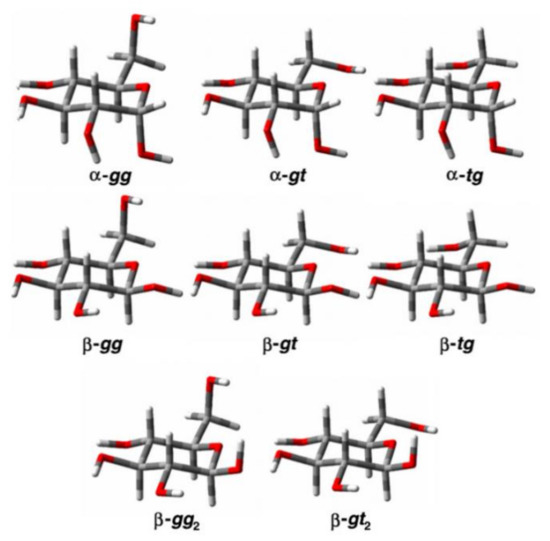
13C nuclear shieldings and proton-proton coupling constants were calculated at the B3LYP/pcJ-2 level for the optimized structures of α- and β-D-glucose. Those structures were averaged in solution for a set of three conformers of α-D-glucose (α-gg, α-gt, and α-tg) and five conformers of β-D-glucose (β-gg, β-gt, β-tg, β-gg
2, and β-gt
2) shown in , which were established earlier by Da Silva and coworkers [
112].
Figure 11. The conformers of α- and β-D-glucopyranose. Reproduced from Roslund et al. [
111] with the permission of Elsevier.
The correlation between the population weighted averages of the calculated
1H-NMR chemical shifts and corresponding experimental values given in reached at in that study [
111] was surprisingly good considering the fact that solvent effects were not taken into account in the geometry optimizations and NMR calculations. The largest Mean Absolute Deviations (MAD) between calculated and experimental values of
1H-NMR chemical shifts were less than 0.25 ppm (7%), and the overall mean absolute deviation was only 0.1 ppm (2.6%). Although the calculated
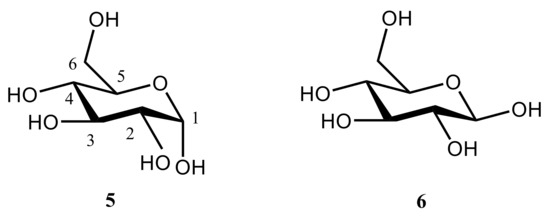
13C-NMR chemical shifts correlated well with the experimental values, the calculated chemical shifts of α and β anomers of D-glucopyranose shown in (accordingly,
5 and
6) were about 10 ppm (some 13–14%) larger than corresponding experimental values.
Figure 12. Anomeric forms of D-glucopyranose: α-anomer (left); β-anomer (right).
Table 1. Comparison of calculated and experimental values of δ
H (ppm), δ
C (ppm), and
JH,H (Hz) of the α and β anomers of D-glucopyranose. Compiled from Roslund et al. [
111].
Much later, related studies were performed for some other monosaccharides, exemplified with D-galactose studied recently by Zrelov et al. [
113]. The latter was shown to undergo interconversion of two hexopyranose forms of D-galactose,
7a and
7b, its two pentofuranose forms,
7c and
7d, and an open-chain intermediate
7e, as illustrated in
Scheme 2.
Scheme 2. Mutarotation of D-galactose studied by Zrelov et al. [
113]. Among monosaccharides, glucose plays an outstanding role due to its unique fundamental properties which are outlined below: (a)—aqueous solutions of glucose undergo mutarotation, leading to a mixture of α- and β-anomers with the heavily overlapping
1H and
13C-NMR spectra; (b)—its nonexchangeable protons lie in rather similar chemical environments and therefore give rise to a crowded
1H and
13C-NMR spectra which are difficult to interpret; and (c)—most importantly, hydroxyl groups of glucose anomers are located in such a way that an effective intramolecular hydrogen bonding is possible [
114].
Keeping this in mind, Bagno and coworkers in their scrupulous study of α-D-glucose [
114] performed computation of its
1H- and
13C-NMR chemical shifts in water through the combination of Molecular Dynamics (MD) and DFT calculations. Resulting structures were optimized with a B3LYP functional using Pople’s 6-31G(d,p) or 6-31+G(d,p) and Dunning’s cc-pVTZ or aug-cc-pVTZ basis sets.
Further calculations of
1H- and
13C-NMR chemical shifts were carried out at the same level. The accuracy of the performed calculations was estimated by the authors as 0.1 ppm for protons and 1 ppm for carbons. A good agreement of the B3LYP calculations of
1H and
13C-NMR chemical shifts of α-D-glucose in water with taking into account solvent effects within the IEF-PCM formalism against experiment had been obtained, as illustrated in . However, much better results following from the MD calculations with taking into account solvent effects of water in an explicit way as illustrated in were reached at in the same study [
114].
Figure 13. 1H and
13C-NMR stick spectra of α-D-glucose in water: (
a,
c)—calculated; (
b,
d)—experimental. Calculated spectra were shifted to match the most shielded signals. Reproduced with minor editing privilege from Bagno et al. [
114] with the permission of the American Chemical Society.
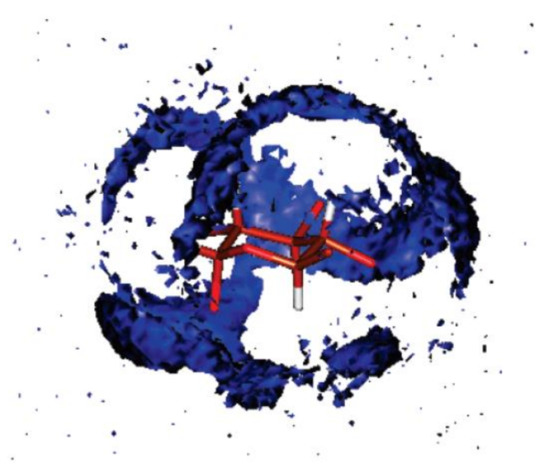
Figure 14. Spatial distribution function of the oxygen of water around glucose representing the probability of finding the oxygen atom of a water molecule at a given point in space around glucose. For clarity, the hydroxyl hydrogen and the hydroxymethylene hydrogen and oxygen atoms have been removed. This view shows the hydroxymethylene carbon and the ring oxygen at the forefront. Reproduced from Bagno et al. [
114] with the permission of the American Chemical Society.
Three years later the same principle authors [
115] continued their studies dealing with the solvation of carbohydrates and performed relevant calculations on α- and β-glucose (
8 and
9, respectively) and α- and β-talose (
10 and
11, respectively) in mixtures of water and acetonitrile. The structure of the solvation shell, obtained by means of molecular dynamics simulation, has been analyzed using radial and spatial distribution functions. In agreement with available experimental data, water was found to preferentially solvate the sugars. The micro-heterogeneity of the mixture, with clusters of hydrogen-bonded water molecules and clusters of the dipole-dipole interacting acetonitrile molecules, favored the solvation of the carbohydrates by the water clusters, as shown in retrieved from that publication.
Figure 15. Snapshots of the solvation shell of four sugars obtained from the MD runs: α-glucose (
8), β-glucose (
9), α-talose (
10), and β-talose (
11). Reproduced with minor editing privilege from Bagno and Saielli [
115] with the permission of the Royal Society of Chemistry.
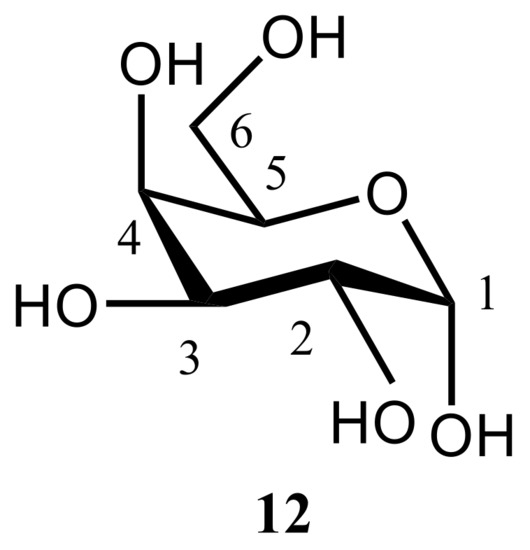
In the related study of the same period of time, Kibalchenko and coauthors [
116] performed first principles calculations together with the solid-state NMR experiments to distinguish between possible hydrogen bonding networks in α-D-galactose
12 (), so to say “in vivo”. Two theoretical models, namely those based on the X-ray structures of Kouwijzer (Model A) and Sheldrick (Model B) represented in were employed in those calculations.
Figure 16. The structural formula of α-D-galactose.
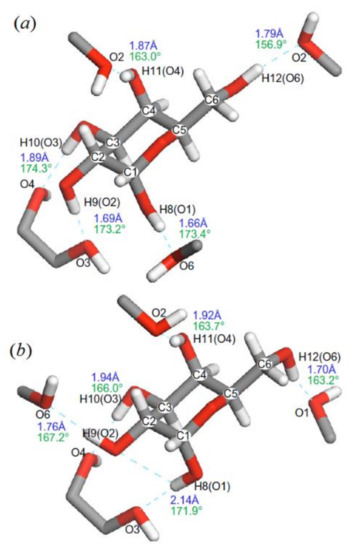
In the latter (Model B), the network was a closed loop in which the longest hydrogen bond (2.13 Å) involved anomeric hydroxyl O1−H linked to the O3 atom, and the shortest (1.70 Å) linked the primary alcohol O6−H to the anomeric oxygen O1. In Model A, the O1‒H∙∙∙O6 hydrogen bond was still the longest (2.02 Å), but O1 was not a hydrogen bond acceptor, and the O1‒H∙∙∙O6 distance (1.69 Å) was the shortest in the hydrogen bonding network.
All
13C-NMR shielding parameters were calculated using the Gauge Including Projector Augmented Waves (GIPAW) approach, see original paper by Pickard and Mauri [
117]. GIPAW is a DFT based method used to calculate magnetic resonance properties, exploiting the full translational symmetry of crystals. In this method, the use of pseudopotentials and plane waves provides an excellent balance of speed and accuracy. GIPAW presents a theory for the all-electron magnetic response within the pseudopotential approximation and is well known in the solid state NMR for its application to the calculation of the first-principles NMR chemical shifts. A Hamiltonian constructed using GIPAW has the required translational invariance in the presence of a magnetic field. In the original paper [
117], the gauge-including projector augmented wave method was applied when describing the original projector augmented-wave method and extending it to the case of a uniform applied magnetic field. In fact, GIPAW is an extension to the projector augmented-wave method, which is valid for systems in the nonzero uniform magnetic fields in the gauge-including projector augmented-wave approximation. Implementation of GIPAW into a parallelized planewave pseudopotential code allows the calculation of NMR chemical shifts in large, low-symmetry extended systems.
As an example, Szeleszczuk and coauthors [
118] demonstrated a convenient method for the indirect crystal structure verification of methyl glycosides using the GIPAW method. In that paper, instead of using various time consuming methods of conformational search, the crystal structures of methyl glycoside acetates were used for the desired crystal structure preparation and optimization. Due to the high sensitivity of chemical shifts to the local atom neighborhood, the method of structure verification based on the GIPAW calculations was found to be very promising. Since performed calculations included both the symmetry and periodicity of the crystal structures, the results depended upon the correct representation of intermolecular interactions, especially hydrogen bonding which is known to be dominant in the crystal structures of carbohydrates with freely rotating hydroxyl groups. It was found that the NMR-based method of crystal structure verification could provide information about the fragments of carbohydrate that need to be reoriented in order to obtain the correct crystal structure. Finally, in spite of not taking into account temperature effects for the correct structures, the correlations between experimental and theoretical chemical shifts were found to be very reliable. It was thus concluded that temperature effects were not essential in the case of methyl glycosides, which may not be so in the case of other classes of saccharides.
Figure 17. α-D-galactose with hydrogen atom positions optimized with the PBE exchange-correlation functional: (
a)—Kouwijzer’s model (Model A) and (
b)—Sheldrick’s model (Model B). The hydrogen bond networks are shown by dashed lines with calculated bond length (blue) and O–H∙∙∙O bond angles (green). Reproduced from Kibalchenko et al. [
118] with the permission of Elsevier.
Coming back to the paper by Kibalchenko and coauthors [
118], a partial geometry optimization in that study was carried out prior to calculating the NMR parameters. The isotropic chemical shifts (represented here with
13C-NMR chemical shifts in ) were obtained from the isotropic average of the shielding tensors evaluated with the PBE and KT3 functionals. Based on those results, it was concluded that the Kouwijzer structure for α-D-galactose (Model A) was much closer to experiment, as compared to Model B.
Table 2. 13C-NMR isotropic chemical shifts of α-D-galactose (
12) calculated using the PBE and KT3 functionals. Compiled from Kibalchenko et al. [
117].
Also, as was commented by one of the referees, for the calculation of the NMR parameters of carbohydrates in solid state, some more specific functionals (other than those used for the liquid state calculations) have been proven to be significantly more accurate, such as PBE with dispersion correction (PBE + TS).
For the nuclei with a spin of more than 1/2 exhibiting a quadrupole moment, the Electric Field Gradient (EFG) occurs, which results in an essential peak broadening in the solid-state NMR spectra. In this case, the EFG calculations should be performed in order to determine the electric field gradients for each atom in the system, especially in terms of the quadrupole interactions. Indeed, “a quadrupolar nucleus is efficiently relaxed by a non-uniform electric field that is a product of the solute molecules interaction with the dipolar solvent. This relaxation is dependent on the interaction of the electric field gradient at the nucleus. When the nucleus is in a molecule that is surrounded by a non-spherical electron density distribution, it creates a gradient. The field gradient,
q, describes the electron charge cloud’s deviation from spherical symmetry. The value of
q is found to equal zero if the groups around the quadrupolar nucleus have a cubic symmetry, such as in the T
d point group. However, if a non-cubic molecule has a threefold or higher symmetry axis, the deviation from spherical symmetry is expressed as a magnitude of
q. The two parameters,
q, the field gradient, and η, the asymmetric parameter, become necessary only if the molecule’s point group’s highest symmetry axis is a threefold symmetry or less. Depending on the molecule, certain cancellations can take place leading the asymmetric parameter, η, to equal zero. This is caused by a combination of very specific bond angles and charge distribution in the molecule being analyzed. Ultimately, the effectiveness of the relaxation is dependent on the magnitude of the electric field gradient,
q. Linewidth broadening in the NMR spectrum is consequential of the rapid nuclear quadrupole relaxation of the quadrupole nucleus. Consider an analogous situation: chemical exchange. It is known that when the nuclei’s spin state rapidly changes it causes broadening in the spectrum. Similarly, the nuclear quadrupole relaxation rates of a quadrupolar nucelus corresponds to an intermediate rate of chemical relaxation.The apparent broadening effect also influences the spectra of the other nuclei attached to the quadrupolar nucleus, including protons. In some cases, the rapid nucleur quadrupole relaxation times (T
1) can cause extensive homogenous broadening (consequential of readily relaxing nuclei) rendering the proton signal of the quadrupolar nucleus completely unobservable in the
1H-NMR spectrum. T
1 is determined by two factors: the electric quadrupole moment (
Q) and the presence of the electric field gradient (
q) across the nucleus. A common approach to resolving quadrupolar effects on the spectra of solution state NMR is elevating temperatures while collecting NMR data. The molecular reorientational correlation times are then shorter than the normal time scale, so the homogenous broadening of the line can be reduced. Unfortunately, the temperature required to create this motional tapering is unfeasibly high for many samples that would deem this technique necessary” [
119].
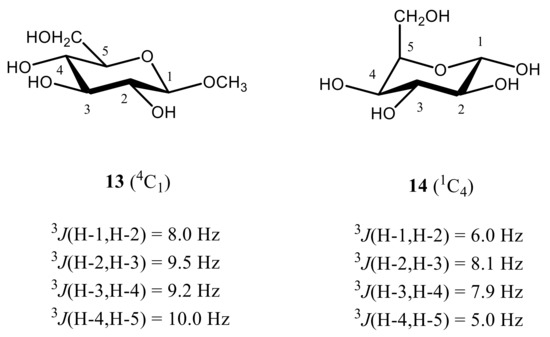
Coming to the calculation of 1H-1H spin-spin coupling constants of monosaccharides, it should be recalled that their stereochemical applications were historically limited to the implementation of four vicinal proton-proton couplings, namely, 3J(H-1,H-2), 3J(H-2,H-3), 3J(H-3,H-4), and 3J(H-4,H-5), which are most critical to the conformation of the hexapyranosyl moiety. One of the most illustrative examples of stereochemical dependence of these coupling constants is the assignment of a highly preferred 4C1 conformation of methyl β-D-glucopyranoside in aqueous solution (see below). This assignment was based on the magnitudes of the most informative vicinal proton-proton coupling constants of the pyranose ring, calculated theoretically and compared to experiment.
As follows from numerous calculations and experimental measurements, all of those couplings are essentially large (8 Hz or larger), which indicates on the fact that all coupled protons are in the axial positions of the pyranose ring characterized by their mutual transoidal orientations. Such a behavior markedly contrasts to that of α-D-idohexopyranose, where corresponding 3JH,H couplings range from about 5 to 8 Hz, which suggests a highly preferable alternative 1C4 conformation, as compared to a classical 4C1 one. The latter is characterized by a highly energetically unfavorable axial orientation of all four hydroxyl groups at C-1, C-2, C-3, and C-4 positions of the pyranose ring and equatorial orientation of the CH2OH group at C-5. On the contrary, in the alternative 1C4 conformation of all four hydroxyl groups occupy favorable equatorial orientations while only CH2OH group is in the unfavorable axial orientation, as illustrated below for methyl β-D-glucopyranoside (13) and α-D-idohexopyranose (14), see .
Figure 18. Most informative vicinal proton-proton spin-spin coupling constants in the representative β-D-glucopyranoside (
13) and α-D-idohexopyranose (
14), providing accordingly,
4C
1 and
1C
4 conformations. Adopted from the review [
1].
It thus follows that in monosaccharides, four 3JH,H couplings are especially sensitive to the pyranosyl ring conformation, namely 3J(H-1,H-2), 3J(H-2,H-3), 3J(H-3,H-4), and 3J(H-4,H-5), which is mostly representative for the considered β-D-glucopyranoside providing mostly 4C1 conformation and α-D-idohexopyranose existing mainly in the alternative 1C4 conformation.
In some cases, geminal, vicinal, and long-range (over more than three bonds) 1H-1H spin-spin couplings can be utilized for this purpose based on their structural and conformational dependencies, which can be used, as an example, in the conformational analysis of O-glycosidic linkages in polysaccharides. In total, as many as 68 one-bond, geminal, and vicinal coupling constants (namely, 14 nJCC, 41 nJCH, and 13 nJHH,) were reported for β-D-glucopyranoside, which redundantly describe configuration and conformation of the glucopyranosyl moiety. However, only four most informative proton-proton couplings are usually employed in the stereochemical analysis of carbohydrates, while the rest of those coupling constants are used only sporadically.
It should also be noted that
2JH,H couplings are affected by the conformation of the C–O bond adjacent to the exocyclic hydroxymethyl carbon while
3JH,H are strongly influenced by the relative arrangement of the hydroxyl groups attached to carbons bearing coupled hydrogens [
120,
121,
122]. In some pyranosyl ring conformations, longer-range four-bond
1H-
1H coupling constants depend on the geometry of the corresponding four-bond H-C-C-C-H coupling path [
123] as well as on the relative arrangement of hydroxyl groups along the coupling pathway, which was well reproduced in the performed DFT calculations. These trends provided an additional guiding thread to the conformational behavior of the pyranose ring.
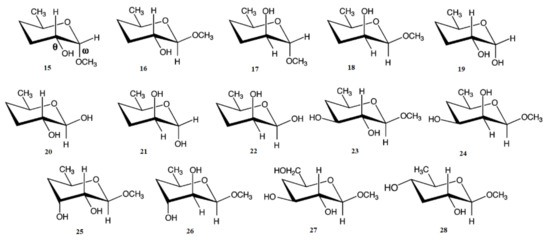
In the earlier classical study in this area, Zhao and coworkers [
124] performed a comprehensive theoretical study of the dihedral angle dependences of vicinal
1H-
1H spin-spin coupling constants based on their pronounced Karplus dependences in the series of model compounds
15–28 shown in
Scheme 3, representing methyl α- and β-D-aldohexopyranosides and corresponding α- and β-D-aldohexopyranoses. The detailed analysis of calculated one- and two-dimensional dihedral angle dependences of vicinal
1H-
1H spin-spin coupling constants in these compounds performed in that study at the DFT level are presented in ; . Those results demonstrated that
3JHCOH depended primarily on the H-C-O-H dihedral angle and that it was essentially unaffected either by the conformation at the adjacent C−O bond or by the type of the carbon atom bearing hydroxyl group.
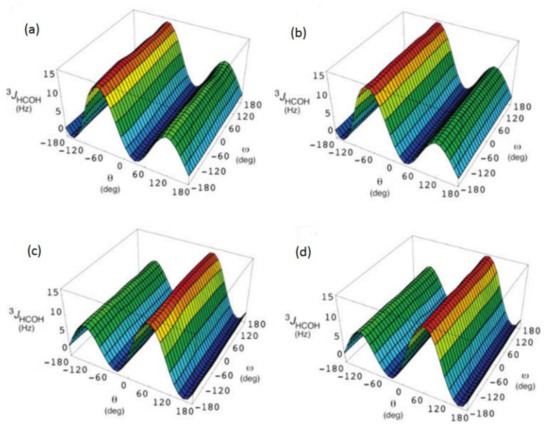
Figure 19. Dependences of calculated
3J(H-2,O-2H) on the dihedral angles ω and θ in (
a)—compound
15, (
b)—compound
16, (
c)—compound
17, and (
d)—compound
18. Reproduced from Zhao et al. [
124] with the permission of the American Chemical Society.
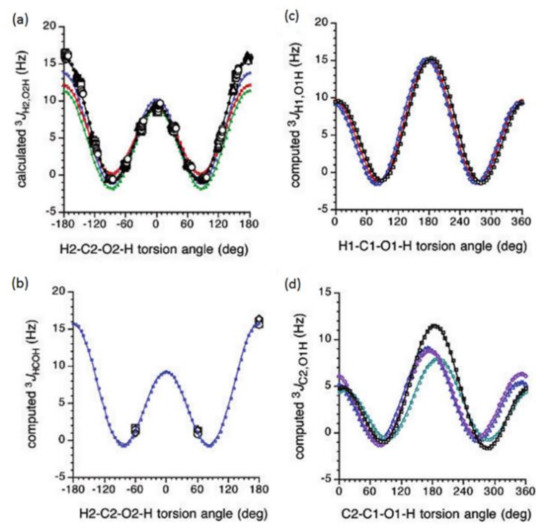
Figure 20. Dihedral angle dependences of vicinal
1H-
1H spin-spin coupling constants in model compounds chosen to mimic methyl α- and β-D-aldohexopyranosides: (
a)—Dependence of
3JH2,O2H on the H
2-C
2-O
2-H torsion angle θ in
15–
18; (
b)—Dependence of
3JH2,O2H on the H
2-C
2-O
2-H torsion angle in
15–
18; (
c)—Dependence of
3JH1,O1H on the C
1-O
1 bond torsion in
19–
21 (blue triangles) and
20–
22 (black squares); (
d)—Dependence of
3JC2,O1H on the C
1-O
1 torsion angle in
19 (green circles),
20 (blue triangles),
21 (black squares), and
22 (purple diamonds). Reproduced with minor editing privilege from Zhao et al. [
124] with the permission of the American Chemical Society.
In general, those findings provided an essential support for the use of a generalized Karplus equation to interpret
3JHCOH coupling constants in the broad scope of saccharides. However, it was found that separate equations were needed to treat
3JH1,O1H because those couplings were subjected to the additional effect of the internal electronegative substituents. The latter effect caused phase shifting of the corresponding Karplus curves, which was due to the nonequivalent values of the gauche couplings. It was demonstrated that
3JH,H-based analysis of H
1−C
1−O
1−H dihedral angles was very likely to reflect glycoside conformations. Furthermore, a high presence of the OH groups in those structures resulted in the unique chemical and physical properties of those compounds due to their ability to form intra- and intermolecular hydrogen bonds, which should lead in the final diagnosis to a more straightforward analysis of their C−O rotamer populations [
124].
Scheme 3. Model compounds chosen to represent α- and β-D-aldohexopyranoses and methyl α- and β-D-aldohexopyranosides. Reproduced from Zhao et al. [
124] with the permission of the American Chemical Society.
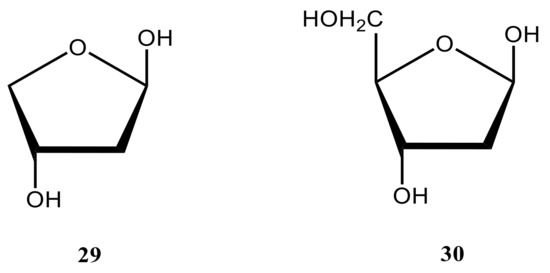
Coming to the substituent and stereochemical effects on carbon-hydrogen coupling constants in carbohydrates, it should first be recalled that the one-bond couplings drastically depend on their stereochemical structure and, on the other hand, on carbon
s-character or the length of the corresponding carbon-hydrogen bond [
125]. Thus in the cited paper, one-bond
13C-
lH coupling constants have been studied in the furanose cycle of 2-deoxy-β-D-glycerotetrofuranose (
29), a restricted model of 2-deoxy-β-D-erythro-pentofuranose (
30) met in DNA, as a function of ring geometry in order to assess their utility as conformational probes. The ab initio calculations were conducted in that study on ten envelope forms and the planar form of
29 (modelling that of
30) () at the MP2 level with Pople’s 6-31G(d) basis set. Computed
1JCH values were found to be sensitive to the orientation of the C‒H bond, with larger couplings observed when a C‒H bond occupied the quasi-equatorial position.
Figure 21. The structural formulas of 2-deoxy-β-D-erythro-pentofuranose (30) and its model form (29).
At the same period of time, Podlasek et al. [
126] performed a fundamental survey of carbon-hydrogen coupling constants in the representative series of methyl D-aldopyranosides
31–
44. Those compounds favored
4C
1 conformations for methyl α-D-allopyranoside (
31), methyl β-D-allopyranoside (
32), methyl β-D-altropyranoside (
33), methyl α-D-galactopyranoside (
34), methyl β-D-galactopyranoside (
35), methyl α-D-glucopyranoside (
36), methyl β-D-glucopyranoside (
37), methyl α-D-mannopyranoside (
38), methyl β-D-mannopyranoside (
39), methyl α-D-talopyranoside (
40), methyl α-D-xylopyranoside (
41), and methyl β-D-xylopyranoside (
42). On the other hand, methyl α-D-arabinopyranoside (
43) and methyl β-D-arabinopyranoside (
44) preferred the
1C
4 conformation, see
Scheme 4.
Scheme 4. Representative structures of methyl D-aldopyranosides studied by Podlasek and coworkers [
126].
The general findings of the performed study [
126], exemplified here for the one-bond carbon-hydrogen coupling constants involving C-1 and C-2 in pyranoses, are outlined below.
One-bond carbon-hydrogen coupling constants involving C-1:
One-bond coupling constants 1J(C-1,H-1) of equatorial C−H bonds are about 10 Hz larger than those of axial bonds. Based on that tendency,
One-bond carbon-hydrogen coupling constants involving C-2:
The difference between 1J(C-2,H-2) of equatorial and axial C−H bonds (ca. 3.5 Hz) is much less pronounced than in the case of 1J(C-1,H-1) (ca. 10 Hz), so that those coupling constants are insufficient for a reliable use in assigning configuration at C-2 of aldopyranosyl rings. Geminal couplings 2J(C-2,H-3) fall within two groups, one showing large absolute values of 4.9 ± 0.6 Hz (α-allo, β-allo, β-arabino, α-galacto, β-galacto, and β-gluco pyranosides) which are negative in sign, and the other yielding smaller values of 1.2 ± 0.4 Hz (α-manno-, β-manno-, and α-talopyranosides) being probably positive in sign as well. Vicinal couplings 3J(C-2,H-1) and 3J(C-2,H-3) provide no pronounced Karplus type dependence on dihedral angles which is probably due to the oxygen lone pair effect.
Experimental trends highlighted in that publication [
126] were substantiated by numerous calculations from this group, which were reviewed later [
1,
2].
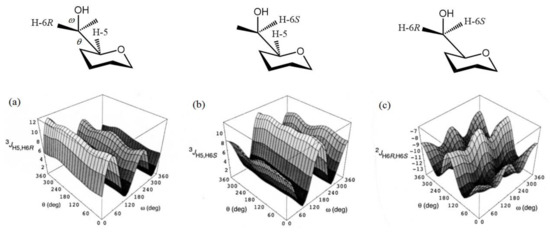
In a more recent paper by Thibaudeau et al. [
127], the dihedral angle dependencies of
2JC,H and
3JC,H in the compound modelling hexopyranoside cycle, (see ) were established at the B3LYP/6-31G(d) level and performed calculations were substantiated by measuring those couplings in the
13C-labeled 4,6-
O-ethylidene derivatives of D-glucose and D-galactose with known constrained dihedral angles. In this respect, methyl gluco- and galactopyranosides
34–
37 shown in
Scheme 4 were prepared as the singly
13C-enriched samples. The latter were used for the experimental measurement of their geminal and vicinal
JC,H and
JC,C couplings involving carbons and hydrogens near to the hydroxymethyl group, as compared to the performed calculations.
Figure 22. Hypersurfaces of ω and θ dihedral angles calculated for (
a)—
3JH5,H6R, (
b)—
3JH5,H6S, and (
c)—
2JH6R,H6S in the restricted compound modelling hexopyranoside cycle. Reproduced with minor editing privilege from Thibaudeau et al. [
127] with the permission of the American Chemical Society.
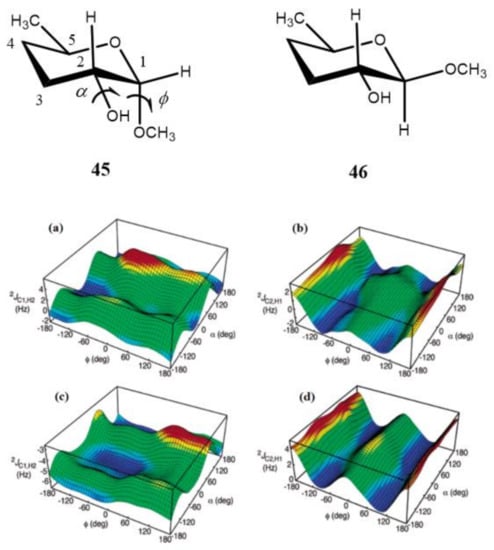
It was found by the same principal authors [
128] that
2J(C-2,H-1) of the aldopyranosyl ring in carbohydrates displayed a systematic dependence on the dihedral angles in glycosides regardless of the relative configuration at C
1 and C
2. This finding followed from the computed hypersurfaces in the model compounds
45 and
46 showing the dihedral angle dependences of geminal carbon-hydrogen couplings presented in . It was also demonstrated that the effect of the C–O bond rotation on
2J(C-1,H-2) and
2J(C-2,H-1) of the aldopyranosyl rings depended significantly on the oxygen lone pair orientation providing essential stereochemical dependence. In general, reported trends in carbon-hydrogen coupling constants of pyranoses suggested their important application to the conformational studies of the glycosidic C–O torsion angles of saccharides.
Figure 23. Computed hypersurfaces showing dependencies of geminal carbon-hydrogen couplings on the dihedral angles φ and α for: (
a)—
2J(C-1,H-2) and (
b)—
2J(C-2,H-1) in compound
45; (
c)—
2J(C-1,H-2) and (
d)—
2J(C-2,H-1) in compound
46. Reproduced from Klepach et al. [
128] with the permission of the American Chemical Society.
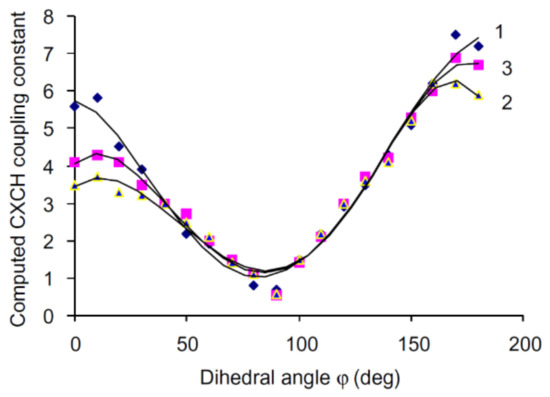
It has long been established that vicinal carbon-hydrogen couplings followed a well-known Karplus dependence on the dihedral angle. To test this dependence between
3JC,H and torsion angle around the anomeric center of carbohydrates, the conformations of 24 derivatives of glucose and galactose were scrutinized by Tafazzoli and Ghiasi [
129]. The authors performed calculations of
3JC,H in that series at the B3LYP level using the 6-311++G(d,p) basis set with taking into account solvent effects of water within the IEF-PCM approach. It was found that those couplings provided a classical Karplus-type dependence with a well-defined minimum at φ ≈ 90°, as illustrated in for glycosides,
O-glycosides and thioglycosides.
Figure 24. Dihedral angle dependence of vicinal carbon-hydrogen coupling constants calculated at the B3LYP/6-311++G(d,p) level in (
1)—
O-glycosides; (
2)—thioglycosides; (
3)—glycosides. Reproduced from Tafazzoli and Ghiasi [
129] with the permission of Elsevier.
Carbon-carbon coupling constants over one and two bonds (
1JC,C and
2JCCC) were shown to be sensitive to the presence and orientation of the hydroxyl groups attached to the coupled carbons depending on the rotation around central C–C bond, demonstrating their well-defined Karplus-type dependence [
130]. The latter originates in the rotation around both C–O bonds reflecting the lone-pair orbital interactions with the C–C bond, which was essentially stronger than the C–C bond rotational effect. A significant new finding of that study was that
1JC,C depended on the internal rotation around the C–O bond, reaching maximum when the hydroxyl proton was anti to carbon atom and a minimum in gauche conformation.
On the other hand, the
2JCCC values in a HO–C–C(OH)–C–OH coupling pathway in aldopyranosyl rings depended markedly on the relative orientations of the hydroxyl groups attached to the terminal coupled carbons (configurational effect) [
131] and on the orientation of a hydroxyl substituent attached to the central carbon (conformational effect) [
132]. In the former paper, interpretation of
2JCCC values was performed and stereochemical behavior of
2JCOC has also been examined. That study provided results which were found to be useful in the conformational analysis of the O-glycosidic linkages in oligosaccharides. Performed calculations of
2JC,C values in compounds modelling aldopyranosyl rings [
131,
132] were found to be in a fair agreement with available experiment.
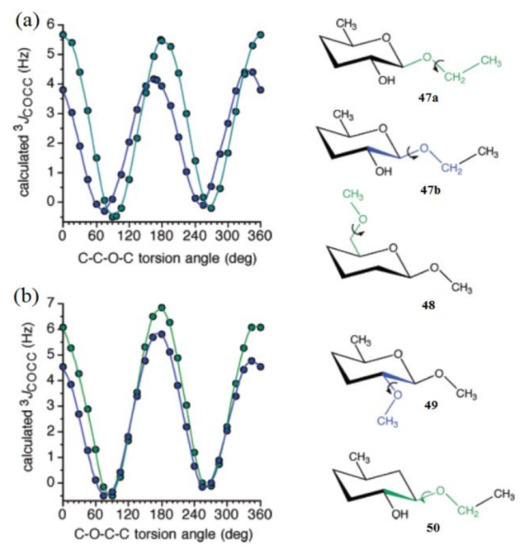
Much later, Zhao et al. [
133] calculated the dependence of
3JCOCC on the central bond torsion angle in the model structures
47–50 shown in . For structures
47a and
47b, the overall shapes of the two curves were the same, but two differences were noteworthy: curve amplitude was slightly reduced for compound
47b, and more importantly, the curve was phase shifted to the left, yielding maximum at about 165°, as compared to the dihedral angle of 180° for compound
47a (a). The C−O−C−C coupling pathway in
3 involved an exocyclic primary alcoholic carbon, whereas that in
4 corresponded to an alternate pathway involving C
1-C
2 cyclic bond, and both curves showed a coupling maximum at about 180° (b).
Figure 25. (
a)—Calculated
3JCOCC values in compounds
47a (green symbols) and
47b (blue symbols) as a function of the central C–O bond torsion. Pertinent coupling pathways are highlighted in green in
47a and blue in
47b. (
b)—Calculated
3JCOCC values in compounds
48 (green symbols) and
49 (blue symbols) as a function of the central C−O bond torsion. The corresponding coupling pathways are highlighted in green in
48 and in blue in
49. Reproduced with minor editing privilege from Zhao et al. [
133] with the permission of the American Chemical Society.
Concerning the C−O−C−C coupling pathways, the curve amplitude was found to be slightly larger for compound 48 as compared to 49. An illustrative comparison was made between the structures 47b and 50 where both coupling pathways were identical, except that in 50 the ring oxygen was absent, and thus the pathway lacked an internal electronegative substituent. The corresponding curve for compound 5 displayed a maximum at about 180°, as was observed also for 47a, 48, and 49, which provided evidence that internal electronegative substituents were responsible for the observed phase shifting.
More recently, Bose-Basu and coauthors [
134] performed a systematic study of stereochemical behavior and structural factors of the one-bond, geminal, and vicinal carbon-carbon coupling constants in the series of aldohexopyranoses and aldohexopyranosides, exemplified below with the representative coupling pathways (related to the corresponding coupling constants) in methyl α-D-glucopyranoside, (
Scheme 5). All calculations were performed at the DFT level with a classical B3LYP functional using the original [5s2p1d|3s1p] basis set [
135] on B3LYP/6-31G(d) geometries.
Scheme 5. Representative
1JC,C,
2JC,C,
3JC,C, and
3+3JC,C coupling constants in methyl α-D-glucopyranoside. The corresponding coupling pathways are shown in red and in red and blue for the dual-pathway coupling constants. Reproduced with minor editing privilege from Bose-Basu et al. [
134] with the permission of the American Chemical Society.
In the course of that study [
134] it was found that generally geminal couplings,
2JC,C, depended on the orientation of the C‒O bonds attached to the terminal coupled carbons. At this,
2JCCC coupling constants (those transmitted across carbon atom) were shown to be also affected by the intervening carbon origin and, on the other hand, by the internal rotation around the C−O bond. Vicinal
3J(C-1,C-6) and
3J(C-3,C-6) couplings demonstrated a classical Karplus-type dependence, but also were affected by the in-plane terminal hydroxyl substituents. For both types of couplings, rotation around the terminal C
5-C
6 bond affected vicinal coupling due to the alternating in-plane and out-of-plane oxygen atom O
6. The values of
3J(C-3,C-6) were shown to be dependent on the configuration at the C
4 atom. Both vicinal couplings,
3J(C-1,C-6) and
3J(C-3,C-6), were influenced by the remote stereochemical effects involving C
1 and C
3 atoms. As a general result of that survey, new structural correlations have been found for
2J(C-3,C-5), which, like
3J(C-3,C-6), demonstrated a “remote” dependence on the configuration at the anomeric center. Finally, investigation of the dual pathway
13C−
13C couplings,
3+3J(C-1,C-4) and
3+3J(C-2,C-5), revealed an important general internal electronegative substituent effect on vicinal carbon-carbon couplings in saccharides.
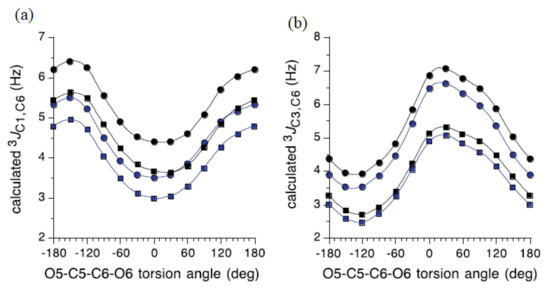
The influence of the C
5−C
6 bond conformation on
3J(C-1,C-6) and
3J(C-3,C-6) for the model carbohydrates
51–54 () reached at by Bose-Basu et al. [
134] is shown in . It was found that
3J(C-1,C-6) was minimal at the O
5−C
5−C
6−O
6 dihedral angle of 0° and maximal at 180°, as shown in a. For this dependence, the curve amplitudes were about 2 Hz, confirming the effect of the O
6 orientation on
3J(C-1,C-6). On the other hand, results of the DFT calculations of
3J(C-3,C-6) shown in b demonstrated that this coupling was maximal at the O
5−C
5−C
6−O
6 dihedral angles of 60° (O
6 in-plane) and -120° (C
4 and O
6 eclipsed). These computational data also revealed the dependence of
3J(C-3,C-6) on the anomeric configuration, with α-anomers yielding slightly smaller couplings, in agreement with the experimental findings. It was also established that configuration at the C
3 carbon influenced the
3J(C-1,C-6) value, while the configuration at C
1 influenced the
3J(C-3,C-6) coupling constant.
Figure 26. Four model carbohydrates used for the establishment of the dihedral angle dependencies of 3J(C-1,C-6) and 3J(C-3,C-6) coupling constants.
Figure 27. (
a)—The effect of the exocyclic CH
2OH conformation on calculated
3J(C-1,C-6) coupling constant in the model carbohydrates
51 (blue circles),
52 (black circles),
53 (blue squares), and
54 (black squares); (
b)—The effect of the exocyclic CH
2OH conformation on calculated
3J(C-3,C-6) values, same symbols. Reproduced from Bose-Basu et al. [
134] with the permission of the American Chemical Society.
Danilova et al. [
136,
137,
138,
139,
140] performed scrupulous SCPT INDO study of the rotational surfaces of
1J1,2 carbon-carbon coupling constants in the series of α- and β-D-mannofuranoses (
55 and
56), α- and β-D-mannopyranoses (
57 and
58), and α- and β-D-mannoseptanoses (
59 and
60), see , , and .
Figure 28. Structural formulas of α- and β-D-mannofuranoses (55 and 56), α- and β-D-mannopyranoses (57 and 58), and α- and β-D-mannoseptanoses (59 and 60).
Figure 29. Rotational surface of
1J(C-1,C-2) of (
a)—α-D-mannofuranose and (
b)—β-D-mannofuranose. Reproduced from the PhD Thesis by Danilova [
136].
Figure 30. Rotational surface of
1J(C-1,C-2) of (
a)—α-D-mannopyranose and (
b)—β-D-mannopyranose. Reproduced from the PhD Thesis by Danilova [
136].
Figure 31. Rotational surface of
1J(C-1,C-2) of (
a)—α-D-mannoseptanose and (
b)—β-D-mannoseptanose. Reproduced from the PhD Thesis by Danilova [
136].
All rotational surfaces were characterized by the well-defined four maxima and four minima, which was due to the fact that rotation of the hydroxyl groups exercised an appreciable effect on
1J(C-1,C-2) coupling constants in the furanose, pyranose, and septanose forms of carbohydrates. Undoubtedly, those dependences originated in the well-known oxygen lone pair effect on
1JC,C [
141], which should be taken into account in the conformational analysis of the cyclic forms of carbohydrates based on the stereospecificity of carbon-carbon coupling constants involving anomeric carbon. Stereochemical dependences of
1J(C-1,C-2) of monosaccharides are illustrated in for the D-forms of the most common hexopyranoses.
Table 3. One-bond spin-spin coupling constants
13C-
13C (Hz) of the α and β forms of D-hexopyranoses calculated at the SCPT INDO level as compared to experiment (given in parenthesis). For structures, see
Scheme 4. Compiled from the PhD Thesis by Danilova [
126].
A nice agreement of calculated couplings with experiment was observed in that study. As one can see in , the range of calculated 1J(C-1,C-2) coupling constants depends on the stereochemical structure of the pyranose ring varying within ca. 5 Hz from 43.4 Hz in α-D-allose to 48.7 Hz in α-D-idose. It should be emphasized that any data on experimental measurement of 1J(C-1,C-2) relates to the equilibrium of the normal and alternative conformers (shifted to one of the forms in each particular case), so that a correct comparison of calculated and experimental values could be performed only when one of the conformers essentially dominates.
4. Di- and Polysaccharides
We start this section with the discussion of the most illustrative example of heparin oligomers. It is well known that heparin, a well-known glycosaminoglycan, is composed of the repeated disaccharide sequences of L-iduronic acid and D-glucosamine linked through the (1→4)-glycosidic bonds. Heparin itself is known mainly for its anticoagulant properties and its biological activity, which is due to its unique pentasaccharide sequence. A number of papers, mainly those produced by Hricovíni and coauthors (which are referenced and discussed below), are devoted to the computational, structural, and conformational studies of heparan sulfate glycosaminoglycans. The latter are the linear polyanions containing dimeric repeating units of hexosamine and uronic acid. In fact, the sequence diversity that characterizes heparin and heparan sulfate is generally due to a series of distinct disaccharides combined in the oligomer unit. Those structures arise from different combinations of twelve possible α-D-glucosamine residues with one of the four possible β-D-gluco- or α-L-idopyranuronic acids. The sequence differences are mainly due to the combinations of sulfated substituents in the 2-
O-, 3-
O-, and 6-
O-endo and -exo positions in the cycles of uronic acid or glucosamine residues. As a matter of fact, heparin itself contains 2-
O-sulfated iduronic acid in excess of 60% of the total uronic acids. Those basic features of heparin oligomers are discussed in much detail in the early review by Guerrini, Hricovíni, and Torri [
142].
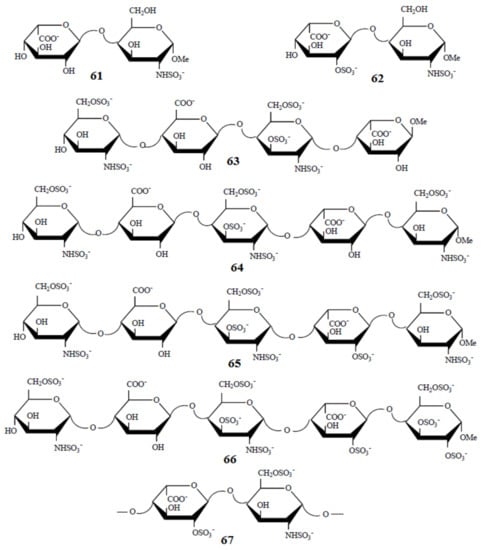
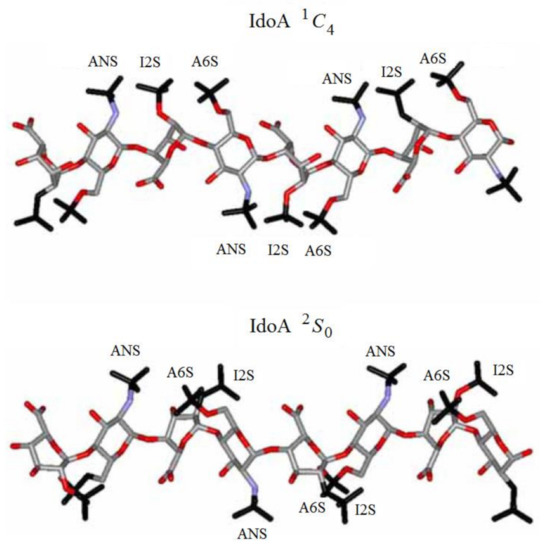
Of particular interest are the heparin oligosaccharides containing idose residue differently substituted and located in distinct sequences, as illustrated in
Scheme 6 for the representative dimeric (
61 and
62), tetrameric (
63), pentameric (
64,
65, and
66), and polymeric (
67) heparin oligosaccharides studied by Hricovini and coauthors. To differentiate those structures, computed and experimental proton-proton coupling constants in the idose residue in several heparin oligosaccharides were established to be highly informative in respect of the structural recognition of different forms of heparin. In the non-sulfated structures, the
3JH,H couplings were found to be relatively small, and this fact was in agreement with the predominance of the
1C4 form. On the other hand, considerably larger couplings supported the preference of the
2S0 form of some heparin oligosaccharides.
Scheme 6. Heparin oligosaccharides containing IdoA residue differently substituted and located in distinct sequences. Reproduced with minor editing privilege from Guerrini [
142] with the permission of Bentham Science Publishers Ltd.
Indeed, a strong influence of the sulfation of the neighbouring residues was evident in the chemically modified oligosaccharides, where conformational equilibrium was shifted nearly completely towards the skewed 2S0 form. On the other hand, in the 1C4 conformation, the sulfate groups are more dispersed, covering a relatively wide range of space on two sides of the molecule. As a result, all sulfate groups in the 2S0 form are arranged into four well-resolved, symmetrically oriented clusters as shown in .
Figure 32. Structural differences of heparin chains associated with distinct conformations of iduronic acid residues. For designations on the figure, see original publication. Reproduced with minor editing privilege from Guerrini [
142] with the permission of Bentham Science Publishers Ltd.
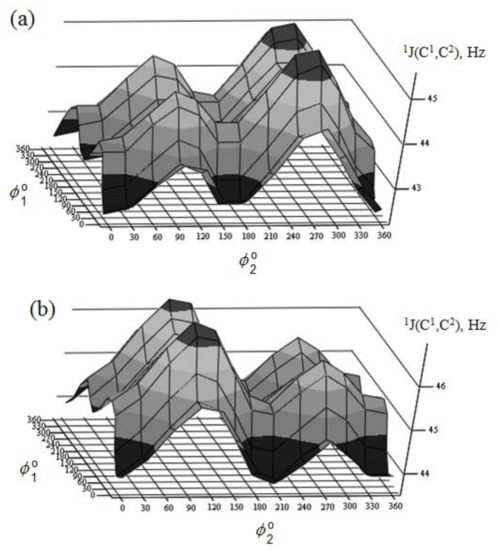
To begin with, we will shortly concentrate on the publications provided by Hricovíni and coauthors on the computational NMR studies of different heparin oligosaccharides. In this line, Hricovíni and coauthors [
143,
144] performed B3LYP/6-311++G(d,p) calculation of vicinal
1H-
1H and one-bond
13C-
1H spin-spin coupling constants of heparin disaccharide, which is methyl
O-(2-deoxy-2-sulfamino-6-
O-sulfo-α-D-glucopyranosyl)-(1→4)-2-
O-sulfo-α-L-idopyranoside uronate tetrasodium salt (
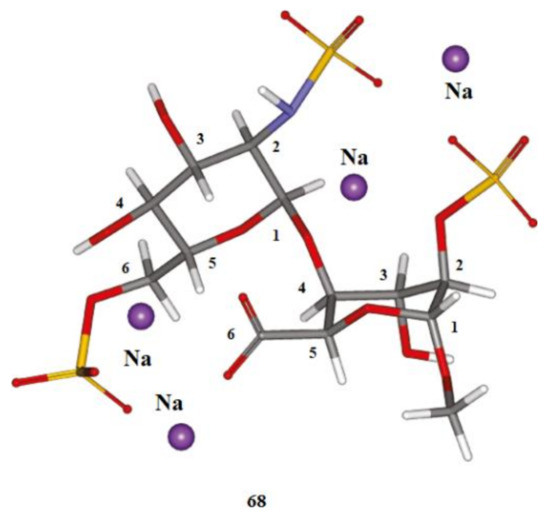
67) shown in . Calculations in that study were performed at the DFT level using B3LYP and M05-2X functionals in combination with Pople’s 6-311++G(d,p) basis set. Solvent effects were treated by the use of multiple water molecules included into computational space in an explicit way. Interesting to note that solvent-induced variations of the computed indirect one-bond carbon-proton coupling constants were found to be of up to 17 Hz (!) between isolated and solvated states. Performed calculations indicated that only two chair forms contributed to the conformational equilibrium of heparin disaccharide.
Figure 33. The structure of heparin disaccharide—methyl
O-(2-deoxy-2-sulfamino-6-
O-sulfo-α-D-glucopyranosyl)-(1→4)-2-
O-sulfo-α-L-idopyranoside uronate tetrasodium salt. Element colors: hydrogen—light gray, carbon—gray, nitrogen—violet, oxygen—red, sulphur—yellow. Reproduced with minor editing privilege from Hricovíni [
144] with the permission of the American Chemical Society.
In the next paper [
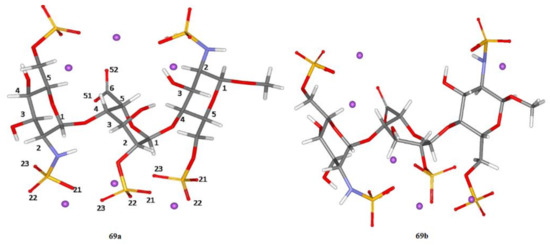
145] from this series, the fully optimized structures of two heparin trisaccharide forms
69a and
69b (differing from each other in the conformation of the central iduronic acid residue) were obtained at the B3LYP/6-311+G(d,p) level, as shown in . In that study, solvent effects were taken into account either via a continuum solvent model or in an explicit way with putting of as many as 57 molecules of water into calculation space.
Reported vicinal proton-proton coupling constants were evaluated with using various functionals (B3LYP and M0-2X) and basis sets (6-311+G(d,p), TZVP, and DGDZVP) and then compared to their experimental values. It followed that the double zeta DGDZVP basis set performed well for most coupling constants and even outperformed (in the meaning of deviation from experiment) the triple zeta TZVP basis set. In fact, this relatively simple basis set provided most satisfactory data, nearly as good as the data obtained by more rigorous approach based on the 6-311+G(d,p) basis set. Computed vicinal proton-proton coupling constants 3JH,H in heparin trisaccharide showed that the best agreement with experiment was achieved when Pople’s 6-311+G(d,p) basis set was employed. Other basis sets, less demanding on computer time and memory, DGDZVP and TZVP, also gave acceptable data for most coupling constants. Performed theoretical analysis showed that stereoelectronic effects considerably influenced the 3J(H-C-C-C) and 3J(H-C-O-C) values. In particular, it was shown that the effect of oxygen lone pairs in the coupling path had a significant influence on 3J(H-C-C-C) and 3J(H-C-O-C) coupling constants differing significantly from each other mainly due to the presence of oxygen in the latter coupling path.
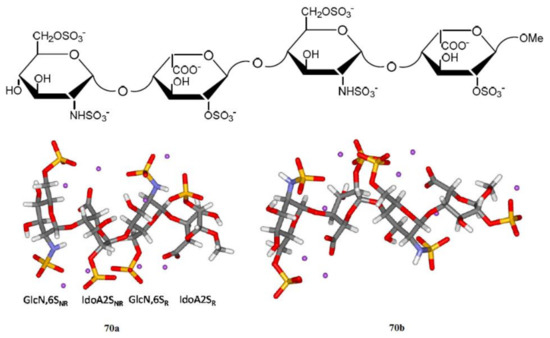
Shown in is a related structure of two forms of heparin tetrasaccharide studied by the same principal author with colleagues [
146,
147]. Performed calculations provided detailed information on molecular structure and spin-spin coupling constants of heparin tetrasaccharide consisting of four units, GlcNS,6S
NR, IdoA2S
NR, GlcNS,6S
R, and IdoA2S
R, representing the predominant heparin repeating-sequence.
Figure 34. Structure of heparin trisaccharide A. The two forms,
69a and
69b, correspond to the different conformations of the IdoA2S residue (central residue). In
69a, the IdoA2S residue is in the
1C4 conformation; in
69b, the IdoA2S residue is in the
2S0 conformation. Both GlcN,6S residues are in the
4C1 conformation. Violet dots represent sodium ions. Solvent (water) molecules are not shown for clarity. Reproduced from Hricovíni and coauthors [
145] with the permission of the American Chemical Society.
Figure 35. The DFT optimized structure of the heparin tetrasaccharide. The two forms,
70a and
70b, have different conformations of the IdoA2S residue. The IdoA2S residues are in the
1C4 conformation in
70a and in the
2S0 conformation in
70b. The GlcNS,6S residues are in the
4C1 conformation. Violet dots represent sodium ions. Solvent (water) molecules are not shown for clarity. The fully optimized molecular structures of two predominant tetrasaccharide conformations of the sulphated iduronic acid residue were obtained at the B3LYP/6-311+G(d,p) level and with applying explicit water molecules to simulate the presence of a solvent. Reproduced from Hricovíni and coauthor [
147] under an open access Creative Commons Attribution 4.0 International Public License. Vicinal proton-proton coupling constants of heparin tetrasaccharide presented in showed that the best agreement of the performed calculations with experiment was obtained with a weighted average of
1C4 and
2S0 conformations of 67:33 for the IdoA2S forms. Detailed analysis of the Fermi-contact terms of
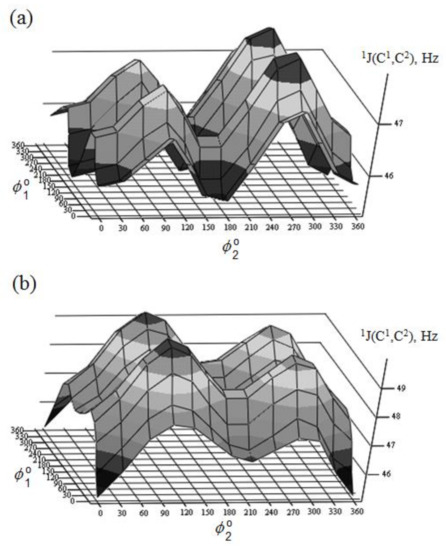
3J(H-C-C-H) demonstrated that important contributions originated from the lone pairs of the neighbouring oxygen atoms. Performed calculations also showed that magnitude of the diamagnetic spin-orbit contributions were sufficiently large as compared to the total values of proton-proton coupling constants.
Table 4. Computed averaged vicinal proton-proton coupling constants (Hz) of heparin tetrasaccharide as compared to experiment. Compiled from Hricovini [
147].
In addition to those results, performed DFT calculations provided insight into the formation of intra- and intermolecular hydrogen bonds and ionic interactions. In particular, it was shown that the H-bond network consisted of intra- and interresidue intramolecular hydrogen bonds that affected the overall molecular structure. Indeed, two interresidue bonds were found in 70a while neither of those bonds was present in 70b.
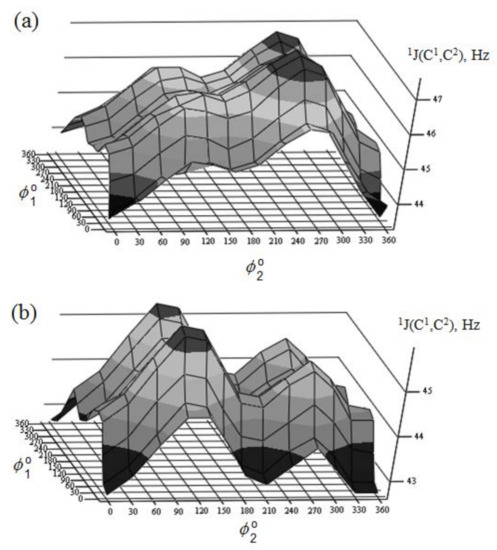
Finally, performed DFT calculations as compared to experiment were applied to the analysis of heparin pentasaccharide (
71) shown in [
148]. In that paper, the fully optimized molecular geometries of two pentasaccharide conformations
71a and
71b (presented in the same figure) were obtained at the B3LYP/6-311+G(d,p) level in the presence of water solvent, the latter included in an explicit form. The averaged vicinal proton-proton coupling constants computed at the DFT level (which are exemplified in ) demonstrated that the best agreement with experiment was obtained with a weighted average of
1C4:
2S0 = 15:85 conformations of the sulfated L-iduronic acid forms indicating that this ratio was shifted towards the
2S0 form.
Figure 36. Chemical structure of heparin pentasaccharide (
71). The IdoA2S residue is in the
1C4 conformation in
71a and in the
2S0 conformation in
71b. Other residues are in the
4C1 conformation. Violet dots represent sodium ions. Solvent water molecules are not shown for clarity. Reproduced from Hricovini [
148] with the permission of the American Chemical Society.
Table 5. Computed averaged vicinal proton-proton coupling constants (Hz) of heparin pentasaccharide as compared to experiment. Compiled from Hricovini [
148].
A detailed analysis of calculated spin-spin coupling constants, including their individual contributions, performed in that study [
146] demonstrated that the Fermi-contact contributions to
3JH,H coupling constants arouse not only from the electrons on atoms along the coupling path, but from the lone pairs of oxygen atoms at the glycosidic linkages as well. It was shown that the spin-orbit contributions to some
3JH,H were comparable in magnitude with their Fermi terms. The values of the diamagnetic spin-orbit terms primarily depended on the contributions including those from molecular orbitals in the neighborhood of the coupled nuclei as well as from the more remote orbitals. For carbon-proton coupling constants, the Fermi-contact terms were the dominant contributions, and the contributions of spin-orbit terms were substantially smaller for most of
3JH,H couplings.
Geometry optimization in the performed study of heparin pentasaccharide [
146] was carried out at the B3LYP/6-311+G(d,p) level, while spin-spin coupling constants were calculated with the same functional and the DGDZVP basis set. The geometry optimization was performed for two conformations of the IdoA2S residue
, whereas all other residues were adopted in the
4C1 conformation. Solvent effects were taken into account in an explicit way for as many as 98 water molecules in view of the fact that hydration of polar groups in heparin pentasaccharide was better described in that way than with a continuum IEF-PCM model. The initial positions of solvating water molecules were based on coordinates of oxygen atoms in water molecules in the published crystal data of sulfated monosaccharides.
The next illustrative example (which is retrieved from the fundamental review [
1]) deals with a marked Karplus dependence of
2J(H-6
R,H-6
S),
3J(H-5,H-6
R), and
3J(H-5,H-6
S) which could be used to establish conformational behavior of a representative disaccharide, methyl β-D-galactopyranosyl-(1→6)-β-D-glucopyranoside (
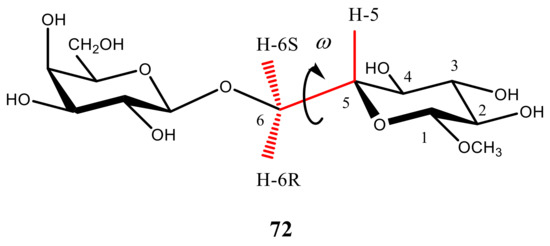
72), , in respect to the internal rotation around its C(5)-C(6) bond.
Figure 37. A representative structure of methyl β-D-galactopyranosyl-(1→6)-β-D-glucopyranoside used for the investigation of the Karplus dependence of 2J(H-6R,H-6S), 3J(H-5,H-6R), and 3J(H-5,H-6S).
Indeed, the fractional populations of rotamers dealing with the internal rotation around the C(5)-C(6) bond of 72 could be easily determined through the Karplus analyses of its 2J(H-6R,H-6S), 3J(H-5,H-6R), and 3J(H-5,H-6S) coupling constants demonstrating marked Karplus-type behavior. In general, the Karplus-type dependences of 2JH,H and 3JH,H in carbohydrates and oligosaccharides enable a straightforward assignment of their rotational conformations based on the DFT calculations of their dihedral angle dependencies in combination with experiment.
Zhang et al. [
149] tried to find the evidence of the intramolecular hydrogen bonding involving the hydroxyl group at the C
3 and O atoms of the adjacent pyranosyl ring in the experimental and the B3LYP computed values of the inter-ring
3hJC-4′,H coupling constant, as exemplified below for methyl α-lactoside methyl β-D-galactopyranosyl-(1→4)-α-D-glucopyranoside (
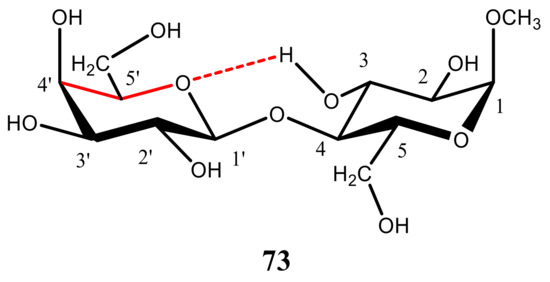
73). However, computed values of
3hJC-4′,H in
73 () and related structures appeared to be next to negligible (ca. 0.1 Hz), and no presence of that coupling was observed experimentally.
Figure 38. A representative structure of methyl α-lactoside methyl β-D-galactopyranosyl-(1→4)-α-D-glucopyranoside used to find the evidence of the intramolecular hydrogen bonding involving the hydroxyl group at the C3 and O atoms of the adjacent pyranosyl ring.
In one of the early publications by Stenuntz et al. [
135], one-bond carbon-hydrogen couplings,
1JC,H, were calculated at the B3LYP/6-31G(d) level to estimate the percentages of the C
5-C
6 and C
6-O
6 trans rotamers in the series of representative disaccharides
74–
76 and trisaccharide
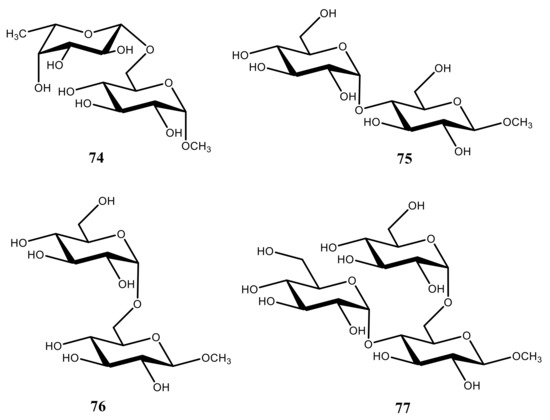
77 (see ). It was found that most of those compounds exhibited a preference for the gauche orientation between O
6 and C
5, and as the steric requirements of the substituent at O
6 increased, the percentage of
trans rotamer had also increased.
Figure 39. Chemical structures of the representative disaccharides 74–76 and trisaccharide 77 providing a preference for the gauche orientation between O6 and C5.
Given the variety of different structural factors influencing
1JC,H in that series, it was not possible to derive an accurate generalized equation relating
1JC,H in hydroxymethyl fragments of the carbohydrate scaffold to specific molecular parameters. However, despite these limitations, semiquantitative equations for both
1J(C-6,H-6) couplings (involving two diastereotopic protons) have been established [
135]. As a result, based on the computed
1JC,H couplings and those relationships, it became possible to foresee how the relevant C−H, C−C, and C−O bond lengths change in carbohydrates and saccharides as a function of their conformation.
In the recent paper by Zhang et al. [
150], aimed at the improving conformational assignments of
O-glycosidic linkages in oligosaccharides, geminal and vicinal carbon-hydrogen coupling constants across the
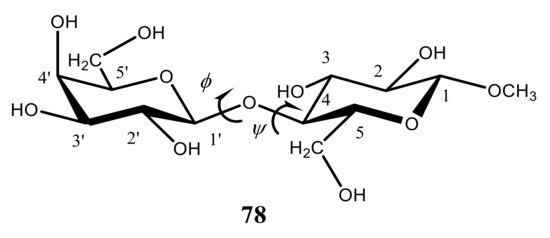
O-glycosidic linkages were investigated at the DFT level. Couplings in a series of twelve structurally related β-(1→4)-linked disaccharides containing systematic covalent modifications in the vicinity of their glycosidic linkages with the most representative methyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (
78) () were thoroughly analyzed by means of the DFT calculations in comparison with experiment. All calculations of those coupling constants were performed with the B3LYP functional, and the effects of solvent water were accounted for within the Tomasi’s IEF-PCM model. In addition, the dependencies of specific trans-
O-glycosidic coupling constants on the geometry of the corresponding coupling pathways were evaluated and parameterized at the DFT level. It was found that conformational behavior of those couplings as a function of φ in the studied series of disaccharides was very much the same. At that, the value of ψ varied significantly, allowing a classification of the studied disaccharides based on the preferred linkage conformation in solution.
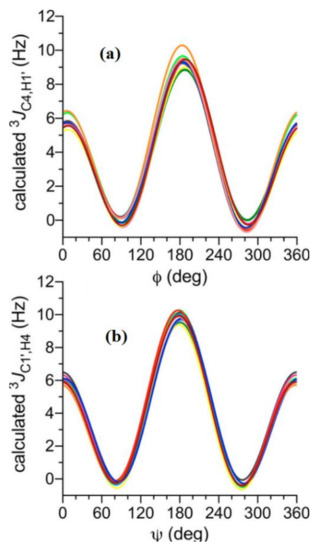
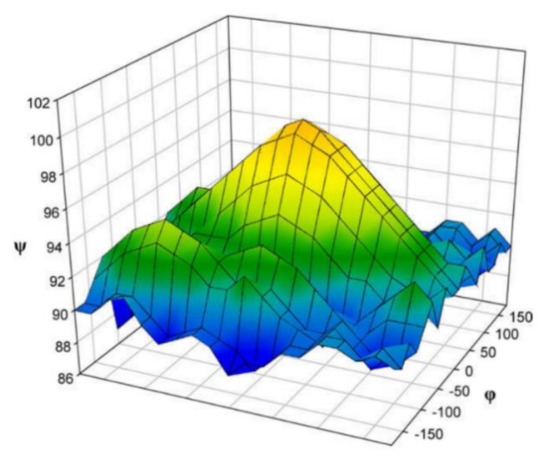
Figure 40. Chemical structure of methyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside.
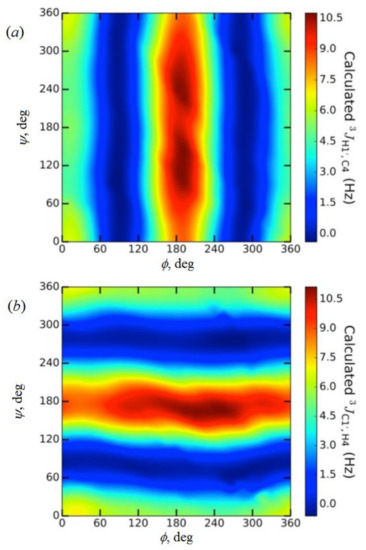
As illustrated in , both trans-O-glycosidic 3JCOCH couplings showed primary dependencies on φ and exhibited dynamic ranges of about 10 Hz each. Visualizations of the DFT data presented in 2D contour plots, as shown for compound 78 in , demonstrated that 3J(C-4,H-1′) was largely unaffected by ψ while 3J(C-1′,H-4) was largely unaffected by φ. It also followed that φ was unaffected by the structural changes in the vicinity of the β-(1→4) linkage, whereas ψ was affected noticeably.
Figure 41. Calculated dihedral angle dependences of (
a)—
3J(C-4,H-1′) and (
b)—
3J(C-1,H-4′) in β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (
78) as a function of φ and ψ. Reproduced from Zhang et al. [
150] with the permission of the American Chemical Society.
Figure 42. (
a)—2D contour plot of calculated
3J(C-4,H-1′) values in β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (
78), showing a primary dependency on φ; (
b)—Contour plot of calculated
3J(C-1′,H-4) values, showing a primary dependency on ψ. Reproduced from Zhang et al. [
150] with the permission of the American Chemical Society.
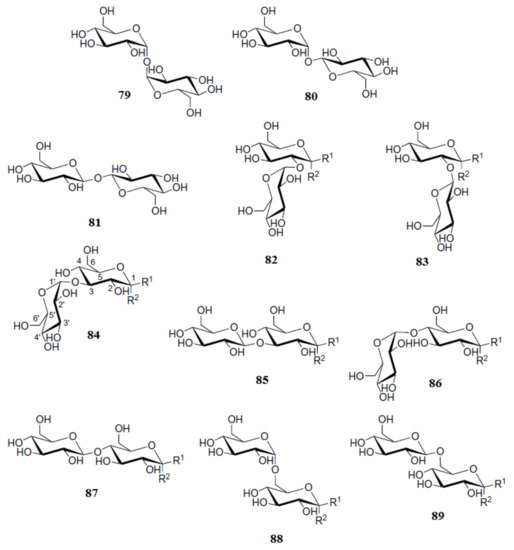
Roslund et al. [
111] performed a combined computational and experimental study of proton-proton coupling constants in α and β anomers of the glucose-based disaccharides
79–
89 shown in a
Scheme 7. In that study, a B3LYP functional was used in combination with Jensen’s triple zeta pcJseg-2 basis set, specially designed for the calculation of nuclear spin-spin coupling constants. The effects of different glycosidic linkage types and positions on the glucose ring conformations and on the α/β-ratio of the reducing end hydroxyl groups were investigated. As a result of that study, rather small differences in
3J(H-4,H-5) coupling constants of the studied disaccharides were observed in the reducing unit of their different anomers. In general, the value of
3J(H-4,H-5) for the α-anomer was found to be more than 10 Hz while for the β-anomer it was less than 10 Hz. Generally, a reasonably good agreement of the performed calculations with experiment was achieved.
Scheme 7. Chemical structures of the glucose-based disaccharides α,α-trehalose (
79), α,β-trehalose (
80), β,β-trehalose (
81), kojibiose (
82), sophorose (
83), nigerose (
84), laminaribiose (
85), maltose (
86), cellobiose (
87), isomaltose (
88), and gentiobiose (
89). R
1 and R
2 stand for H and OH (or either OH and H) in different anomers. Reproduced with minor editing privilege from Roslund et al. [
111] with the permission of Elsevier.
Coming to the discussion of papers dealing with the calculation of
1H and
13C-NMR isotropic chemical shifts and chemical shift tensors measured in solid state of di- and polysaccharides, we will first focus on the methodological survey by Sefzik et al. [
151]. The authors of that paper studied the performance of the HF and DFT methods for this purpose. The quality of the scrutinized methods was assessed by their least-squares linear regression parameters. It was observed that in general DFT outperformed restricted HF theory. For instance, Becke’s functional B3 together with mPW1PW91 provided the best-predicted
13C-NMR shieldings. However, this performance was not universal, as none of the DFT functionals could generally predict saccharide tensors better than the HF theory.
Presented in are the results of the least-squares correlation of calculated
13C-NMR chemical shift tensors for 65 saccharides evaluated using different functionals and basis sets of double and triple zeta quality versus experiment. It is seen that the overall quality of the established linear correlations is very impressive. Thus the RMSD of those correlations were found to be typically 4–5 ppm for the range of about 220 ppm (which is about 2% in relative terms) while the slopes were about (1.0–1.2), which is very close to unit in absolute scale. Interesting to note, the ongoing from the double zeta cc-pVDZ to the triple zeta cc-pVTZ basis set did not improve in general the quality of the correlations established in that paper [
151], as can be seen in the values of RMSD given in the table.
Table 6. Statistical parameters of the least-squares correlation of
13C-NMR chemical shift tensors of 65 saccharides calculated with using different functionals and basis sets versus experiment. Compiled from Sefzik [
151].
It is noteworthy that results reached by Sefzik [
152] and at the same period of time by Sergeyev and Moyna [
152] were critically remarked by one of the reviewers of the presented review as follows: “I would strongly recommend caution and at least a critical remark when including this papers like this in a review. NMR chemical shifts require basis sets with tight exponents and DFT also has different basis set requirements than post HF methods, for which the Dunning basis sets applied by Sefzik have been developed. The fact that HF exhibits the lowest RMSD for the
13C-NMR shifts of 65 saccharides is in stark contrast to practically all relevant benchmark studies on chemical shifts and require a detailed conceptual discussion of all sources or error and an conclusion of how error cancellation leads to these results. The same applies to the discussion of Sergeyev and Moyna’s work (which is discussed next—LBK) using HF and 3-21G and 6-311G(d,p) basis sets”. In my opinion, this criticism is much acquitted.
Sergeyev and Moyna [
152] suggested a novel method for the determination of three-dimensional structure of oligosaccharides exemplified with trehalose, cellobiose, cellotetraose, cellohexaose, cellulose II, and amylose V
6 in solid state. The structures of the examined oligosaccharides are shown in
Scheme 8, while
13C-NMR chemical shift surfaces for the pairs of transglycosidic carbons in α-(1→1′), β-(1→4′), and α-(1→4′)-linked D-Glcp disaccharides as a function of the glycosidic bond dihedrals are presented in . Calculated dihedral angles and
13C-NMR chemical shifts as compared to their equilibrium experimental values are given in . Calculations of
13C-NMR chemical shieldings performed in that study were conducted at the HF/3-21G level. The results obtained for the glycosidic carbons were converted into chemical shifts by subtracting the chemical shieldings computed at the same level of theory for the methyl carbons of a standard—tetramethylsilane, and scaled to the results of the reference HF/6-311G(d,p) calculations. It is seen that generally a very good agreement between theory and experiment has been achieved in that study.
Scheme 8. Chemical structures of trehalose (
a), cellobiose (
b,
n = 1), cellotetraose (
b,
n = 2), cellohexaose (
b,
n = 3), cellulose (
b,
n > 100), and amylose (
c,
n > 100). Reproduced from Sergeyev and Moyna [
152] with the permission of Elsevier.
Figure 43. 13C-NMR chemical shift surfaces for the pairs of transglycosidic carbons in α-(1→1′), β-(1→4′), and α-(1→4′)-linked D-Glcp disaccharides as a function of the glycosidic bond dihedrals. Corresponding surfaces are marked accordingly as (
a–
f). Reproduced from Sergeyev and Moyna [
153] with the permission of Elsevier.
Table 7. Calculated and experimental (in parenthesis)
13C-NMR chemical shifts of different oligosaccharides in solid state. Compiled from Sergeyev and Moyna [
153].
It should be noted that very promising in the structural studies of carbohydrates is the application of molecular dynamics in combination with computational NMR, as exemplified in the paper by Kapla et al. [
153] dealing with the molecular dynamics simulations and NMR spectroscopical studies of trehalose. The authors investigated interactions between lipid membranes and trehalose using NMR spectroscopy and molecular dynamics computer simulations. The dipolar coupling constants derived from theoretical trajectories were compared with those determined experimentally in a weakly ordered solvent and by the solid-state NMR. Analysis of the experimental and MD-derived couplings indicated that the force field used in the simulations reasonably well described experimental results. The signs of dipolar couplings, not available from the experiments on highly ordered systems, were determined from the trajectory analysis. The crucial step in the analysis of residual dipolar couplings was accessed to the conformational distributions derived from the MD trajectories. Furthermore, the conformational behavior of trehalose was investigated in the ordered and isotropic phases and was found to be essentially identical in both cases.
Later, the dissolution mechanism of cellulose in ionic liquids has been investigated [
154] by the variable-temperature NMR using cellobiose and 1-ethyl-3-methylimidazolium acetate (EmimAc) as a model system. It was found that in DMSO-d
6 solution, the hydrogen bonding was formed between the hydroxyl groups of cellobiose and both anion and cation of EmimAc. Based on that fact, it was concluded that both anion and cation had similar roles in the mechanism of cellulose dissolution. However, it was claimed in a forthcoming critical comment [
154] that both experimental and theoretical studies published by different authors indicated that imidazolium ion was not involved in the H-bonding with a sugar solute, as was proposed in the former study.
Esrafili and coauthors [
155] performed DFT calculations of
1H,
13C,
15N, and
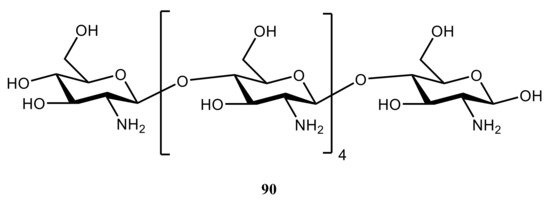
17O chemical shielding tensors in the anhydrous crystalline structure of hexameric chitozan (
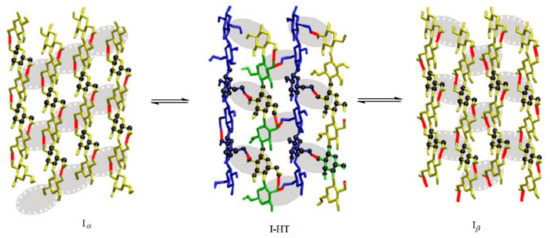
90) (). The hydrogen-bonding effects in cellulose crystalline forms I
α and I
β interconverting to each other via a high temperature intermediate I-HT (see taken from the paper [
156]) were taken into account in that study. The authors of the cited publication [
155] included the most probable interacting molecules with a target molecule in a crystalline phase, the latter considered as a hexameric cluster. In this model, central molecule was surrounded with five other molecules, which participated in the intra- and intermolecular hydrogen bonding with central moiety. The general finding of the performed study [
155] was that intermolecular hydrogen bonding had a major influence on the NMR parameters in isotropic and crystalline states. In addition, the quantum chemical calculations performed with the B3LYP functional and Pople’s 6-311++G(d,p) and 6-31++G(d,p) basis sets indicated that intra- and intermolecular hydrogen bonding played an essential role in the determining the chemical shielding principal components in the molecular frame axes.
Figure 44. Chemical structure of hexameric chitozan.
Figure 45. Interconversion of cellulose crystalline forms I
α and I
β via a high temperature intermediate I-HT. For details, see cited paper. Reproduced with minor editing privilege from Matthews et al. [
156] with the permission of Springer Nature.
Kasat et al. [
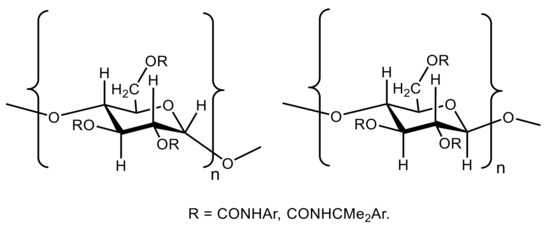
157] studied the effects of the backbone and side chain on the molecular environments in the chiral cavities of three commercially important polysaccharide-based chiral sorbents. They were cellulose tris(3,5-dimethylphenylcarbamate) (known as “CDMPC”), amylose tris(3,5-dimethylphenylcarbamate) (known as “ADMPC”), and amylose tris[(
S)-R-methylbenzylcarbamate] (known as “ASMBC”) with the general formulas of α and β anomers shown in . Those studies were performed using the
13C Cross-Polarization/Magic-Angle Spinning (CP/MAS) and Magic-Angle Spinning (MAS) solid-state NMR together with intensive DFT calculations.
Figure 46. Chemical structures of amylose tris(3,5-dimethylphenylcarbamate) (ADMPC), amylose tris[(S)-R-methylbenzylcarbamate] (ASMBC), and cellulose tris(3,5-dimethylphenylcarbamate) (CDMPC). Amylose has the α-D-(1→4) linkages and cellulose has the β-D-(1→4) linkages.
All NMR chemical shifts for the polymer backbone monomers and dimers were predicted at the B3LYP/6-311+G(d,p) level. It was concluded that molecular environments of the C=O, NH, and phenyl groups showed significant differences in the intramolecular and intermolecular interactions and in the nanostructures of the chiral cavities of those biopolymers. In general, reported results [
157] demonstrated how molecular environments of the chiral cavities did affected their molecular recognition mechanisms, which could be established by the relevent DFT calculations in combination with the solid-state NMR experiment.
Lefort and coauthors [
158] demonstrated that chemical shift surfaces contained information on distributions of various conformations of disaccharides in glassy solid state. The authors found with paying particular attention to the glycosidic linkage atoms in three classical disaccharides, trehalose (
91), sucrose (
92), and lactose (
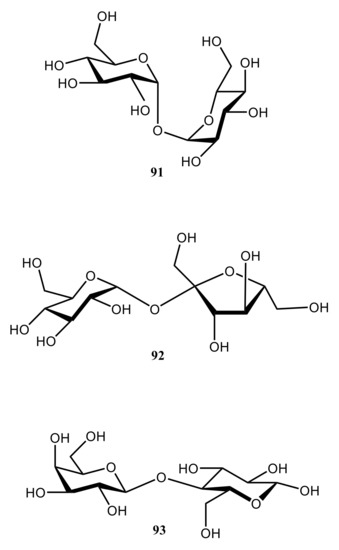
93) (see ), that chemical shift surfaces of glassy disaccharides were almost independent of the method chosen for optimizing their molecular conformations.
Figure 47. Chemical structures of three classical disaccharides, trehalose (91), sucrose (92), and lactose (93).
In particular, a proper consideration of extremes and saddle points of the chemical shift map was correctly accounted for the observed discontinuities in the experimental cross polarization magic angle spinning spectra. It was demonstrated that if those basic requirements were met, the Gauge Including Atomic Orbitals (GIAO) calculated on relaxed conformations lead to a good description of the experimental line shapes. In this approximation, London orbitals include the gauge origin in an exponential pre-factor, removing gauge-origin dependences for some of the integrals and to lowest order. However, it can be rigorously shown that those results are not strictly fully gauge independent.
The results of the performed study [
158] were interpreted in terms of an enhanced flexibility that disaccharides possess in the amorphous solid state, especially on the
13C-NMR time scale. Using amorphous trehalose as a model, the authors considered its most populated conformations in glass state. It was also suggested that ab initio methods were not always necessary for defining the conformational potential basin. Indeed, performed calculations based on the combination of molecular mechanics (MM), semiempirical Austin Model 1 (AM1), and DFT (B3LYP and B3PW91 functionals) with Pople’s basis sets, 3-21+G(d,p) and 6-311+G(d,p), provided the mostly convincing results.
In addition to that study [
158], Shao and coworkers [
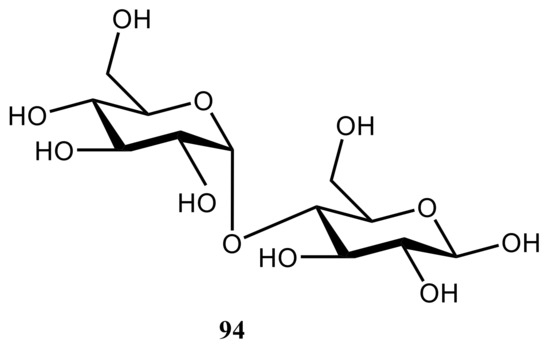
159] investigated the ability of DFT with using the PBE exchange-correlation functional to reproduce experimental principal components for trehalose (
91), sucrose (
92), and maltose (
94), the latter shown in , in solid state and in isotropic phase. A method for assigning poorly dispersed NMR spectra based on comparing experimental and calculated shift anisotropies within the planewave-pseudopotential approach [
160] as well as isotropic shifts has also been described.
Figure 48. Chemical structure of maltose. For the structures of trehalose and sucrose, see .
Different conformations of methyl 3,6-anhydro-4-
O-methyl-a-D-galactoside (
95) and 3,6-anhydro-4-
O-methylgalactitol (
96) together with 3′,4′-dideoxy derivatives of the α-methyl glycoside of carrabiose (
97) and carrabiitol (
98) are shown in
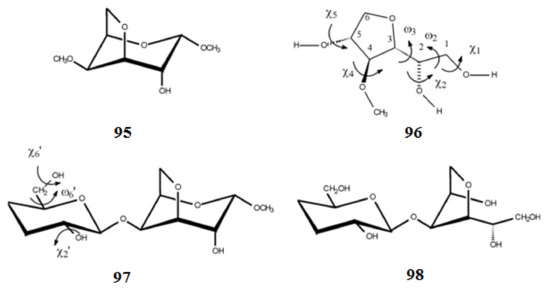
Scheme 9. Those structures were reached at by the molecular mechanics and by quantum mechanical methods at the DFT level of theory using B3LYP functional with 6-31+G(d,p) basis set and at the MP2 level with 6-311++G(d,p) basis set with and without taking into account solvent effects [
161]. In particular, for compound
96, where the five-membered ring was free to move, two main stable conformations were found. Calculation of
1H and
13C-NMR chemical shifts was performed at the same level of theory.
Scheme 9. Chemical structures of methyl 3,6-anhydro-4-
O-methyl-α-D-galactoside, 3,6-anhydro-4-
O-methylgalactitol, and 3′,4′-dideoxy derivatives of the α-methyl glycoside of carrabiose and carrabiitol. Reproduced from Navarro and Stortz [
161] under an open access Creative Commons Attribution 4.0 International Public License.
Suzuki et al. [
162] studied the effects of conformation and hydrogen bonding on the
13C isotropic chemical shifts of the cellobiose units of the native cellulose (
99) at the DFT level. The linear relationship between the chemical shifts of C
4, C
5, and C
6 carbons and the torsion angles around the C−O bonds in the corresponding CH
2OH side groups was found. It is well known that the cellobiose unit (the smallest repeating unit in the polymer) consists of two anhydroglucose units joined through the single-oxygen atoms (acetal linkages) between the C
1 of one pyranose ring and the C
4 carbon of the next ring. This is known as the β-1,4-linkage (for the native cellulose structure, see one of the most comprehensive reviews [
163] and references given therein). Both geometry optimizations and computation of
13C-NMR chemical shifts in that study were carried out at the B3LYP/6-31+G(2d,p) level.
Performed calculations for the cellobiose units in native cellulose revealed the
γH-gauche effect produced by the OH hydrogen atom at the γ position to induce a 3–5 ppm downfield
13C-NMR shift, and this effect was reduced by 2–3 ppm when the intramolecular hydrogen bonding associated with the γ-H atom was formed. The appreciable dependences of the C
1 and C
4 chemical shifts on the torsion angles around the β-1,4-glycosidic linkage were also reported in that paper [
162].
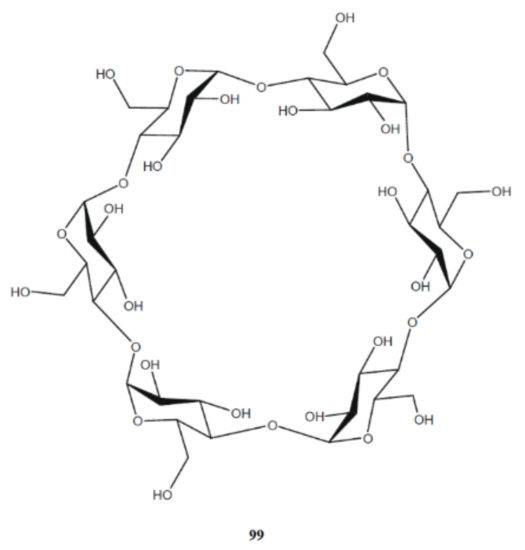
Tafazzoli and Ghiasi [
164] performed a systematic study of the dependence of the anomeric
13C-NMR chemical shift on the glycosidic bond dihedral angles in D-Glcp-D-Glcp disaccharides with the (1→4) linkages in the α-configurations of α-, β-, and γ-cyclodextrins. Those molecules are exemplified here in
Scheme 10 with the structure of α-cyclodextrin shown below possessing six subunits. Cyclodextrins are the cyclic oligosaccharides built up from the α-D-glucopyranose units connected through the α-1,4 glycosidic linkage. The cyclodextrin molecule includes six glucopyranose units, while β- and γ-cyclodextrins consists of accordingly, seven and eight units, arranged in a ring-shape manner. Cyclodextrins have ability to form hydrogen bonds with the surrounding molecules resulting in the formation of a number of inclusion complexes with a variety of inorganic and organic substances.
Scheme 10. The structure of α-cyclodextrin (six subunits). Reproduced from Tafazzoli and Ghiasi [
164] with the permission of Elsevier.
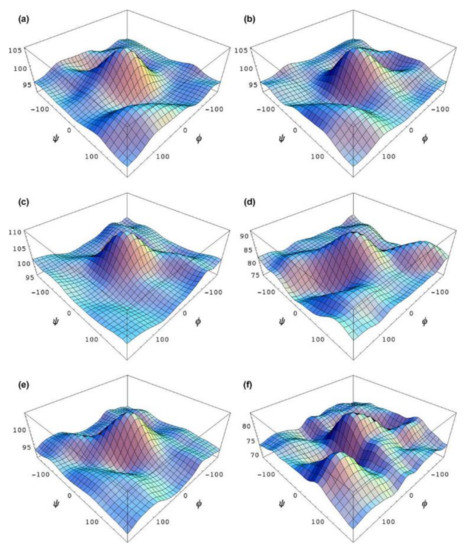
In the course of that study [
164], the chemical shift surfaces versus the dihedral angles φ and ψ for the D-Glcp-α-(1→4)-D-Glcp moiety were computed and plotted as shown in . Based on those results, the empirical formulas in the form of δ(
13C) = ƒ(φ,ψ) were obtained by fitting computational data to the trigonometric series expansions. Those results were consistent with the experimental observations demonstrating the applicability of the
13C-NMR chemical shift surfaces to the study of the conformational behavior of oligosaccharides. Both geometry optimizations and calculations of
13C-NMR chemical shifts were performed at the DFT (B3LYP) and HF levels using Pople’s 6-31G(d) basis set in the IEF-PCM medium (water).
Figure 49. The
13C-NMR chemical shift surfaces of the anomeric carbon of D-Glcp-α-(1→4)-D-Glcp disaccharide. Reproduced from Tafazzoli and Ghiasi [
164] with the permission of Elsevier.
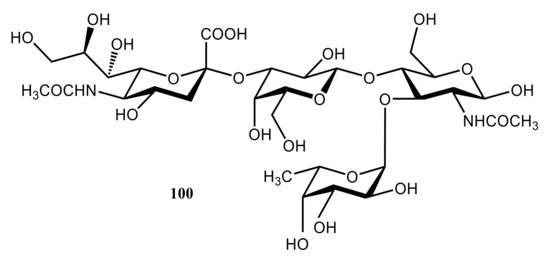
Ishida [
165] performed a computational modeling of the carbohydrate-recognition process in the E-selectin complex with sialyl Lewis X (known as the E-selectin/SLe
x). That complex consists of four sugar components, which are
N-acetylneuramic acid (Neu5Ac), galactose (Gal),
N-acetylglucosime (GlcNAc), and fucose (Fuc) presented by structure
100 (). This model clearly demonstrated that the binding geometries of the E-selectin/SLe
x complex were determined not by a single rigid carbohydrate structure, but rather by the sum of the averaged conformations fluctuating around the minimum free energy region. It was demonstrated that major molecular interactions in
100 were hydrogen bonds between the fucose moiety and the Ca
2+ binding site in the carbohydrate recognition domain, and that it was galactose unit which was important in determining the ligand specificity.
Figure 50. Model structure of the E-selectin complex consisting of four sugar components, which are N-acetylneuramic acid (Neu5Ac), galactose (Gal), N-acetylglucosime (GlcNAc), and fucose (Fuc).
Calculations of 13C-NMR chemical shifts performed at the QM/MM/RHF/6-31G(d)/AMBER level in comparison with experiment revealed most favorable conformations of the E-selectin/SLex complex shown in a. The deviations of calculated chemical shifts from their mean average values were found to be rather small, typically in the range of 0.2–0.4 ppm (as compared to the experimental accuracy of about 0.1 ppm). However, the binding conformation of SLex was clearly not rigid but essentially flexible, especially in the solvent-exposed region. It also followed that the Fuc unit formed stable hydrogen bonds with the Ca2+ binding site while the Gal moiety interacted mostly with the protein.
Figure 51. (
a)—Superimposed geometries of a sialyl Lewis X complex at the carbohydrate-recognition domain. Ten QM/MM optimized structures of SLe
x are superimposed onto one reference structure. Only the SLe
x structures are shown by the stick model. Right panels: (
b)—Experimental NMR profile. (
c)—Computed 1D proton chemical shift profile of the SLe
x ligand. Reproduced from Ishida [
165] with the permission of the American Chemical Society.
It was also demonstrated [
165] that Fuc and Gal residues provided rather small structural fluctuations, while Neu5Ac and GlcNAc, on the contrary, showed much larger molecular motions. By averaging calculated chemical shifts for the QM/MM-refined geometries, the 1D-NMR theoretical spectral profile was obtained (c). In comparing theoretical results with the available experimental spectrum (b), it followed that calculated 1D-NMR profile qualitatively reproduced experimental results within the accuracy of the employed method, QM/MM-RHF/6-31G(d).
The major findings of the performed study [
165] were formulated by the author as follows: “(1)—In the Neu5Ac unit, there are no apparent hydrogen bonds around the carboxyl group, and this unit is more flexible along the glycosidic linkage than are any of the other three sugar units. (2)—In the Gal unit, the 4-OH group is always located near the hydroxyl group of Tyr94, although no apparent hydrogen bonds are formed. The major molecular interactions are the hydrogen bonds between the 6-OH group and the side chains of Glu107 and Lys111. (3)—In the GlcNAc unit, there is no apparent close molecular contact between the hydroxyl groups of the sugar unit and the target amino acid residues. However, the side chain of the NHAc group is relatively flexible, and occasionally forms a hydrogen bond with the side chain of Arg108. (4)—In the Fuc unit, rather stable hydrogen bonds form because of the three types of hydroxyl groups. The 2-OH group can form a moderately strong hydrogen bond with the side chain of Asn83, the 3-OH group occasionally forms a hydrogen bond with Glu107, and the 4-OH group always forms tight and stable hydrogen bonds with Glu80 and Asn82”.
A number of more illustrative examples dealing with stereochemical applications of computational
1H and
13C chemical shifts together with
1H-
1H,
13C-
1H, and
13C-
13C spin-spin coupling constants of carbohydrates can be found in a series of original papers reviewed elsewhere [
1,
2,
7].