Eusocial ants (family Formicidae) engage in a broad range of social behaviors such as nursing the queen’s offspring, foraging for food, and defending their nest. Importantly, these behaviors critically depend on the exchange of information through the detection of chemical cues by a sophisticated olfactory system comprising among the largest number of odorant receptors (ORs) of any insect.
- Hymenoptera
- Formicidae
- ants
- eusocial
- olfaction
- odor coding
- review
1. Introduction
From an evolutionary perspective, ants are an extraordinarily successful insect taxon that are globally pervasive and comprise more than a quadrillion individuals [1]. This success is likely due to the complex eusocial structure and sophisticated olfactory system that drives collective behavior among individuals throughout a colony (Figure 1). Without centralized control, sterile female workers tend to the queen’s offspring (“nurses”), construct and maintain nests (“builders/midden workers”), defend and police the colony (“soldiers”), and search for food (“foragers”). Beyond these fundamental tasks, which are commonly observed across eusocial insect taxa, the social life of certain ant species may be quite extraordinary. Attine ants rely on the collection of leaves that they use as a substrate to maintain elaborate fungal gardens [2]. Army ants create living nests with their bodies, known as bivouacs, where they shelter the queen and store food and brood among the interior chambers [3]. When selecting a new nest site, rock ants engage in a democratic decision-making process that relies on quorum sensing [4]. To accomplish these impressive feats, ants rely largely on sophisticated chemical communication systems that provide an extraordinary degree of discrimination and sensitivity [5].
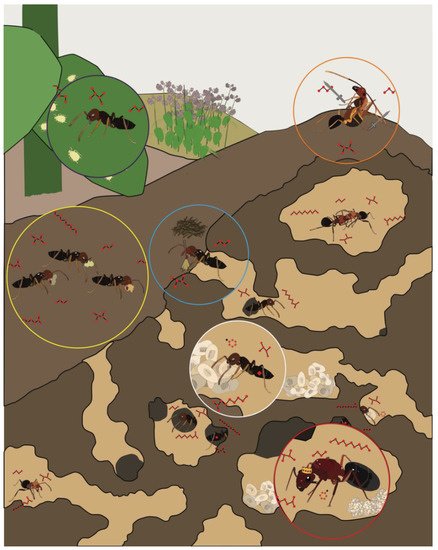
Figure 1. The regulation of complex social behaviors in ant colonies relies on the transmission and detection of chemical information among discrete castes and worker groups, including reproductive “queen(s)” (red circle), brood-care “nurses” (white circle), cleaning “midden workers” (blue circle), food collecting “foragers” (yellow circle), aphid-tending “farmers” (purple circle), and colony defending “soldiers” (orange circle).
Ants communicate with one another by exchanging an array of chemical messages that includes general odorants common to many insects, as well as the social pheromones and other chemical blends that distinguish ants from other, solitary insects. Many of these messages are detected via olfactory signal transduction pathways largely localized to the antennae [5]. Complex blends of cuticular hydrocarbons (CHCs), as an example, are an especially important class of semiochemicals that convey a broad range of social information including colony membership, fertility, and task group [6]. In the course of a brief antennation event, where ants make mutual contact with their antennae, an ant can identify a foraging nestmate or an intruding non-nestmate based on their respective CHC profiles [7,8]. CHCs are produced by oenocytes associated with the fat body [9,10,11], and it is believed that the post-pharyngeal gland plays a central role in storing and distributing the hydrocarbons involved in colony identity [12]. Indeed, there are considerable qualitative and quantitative similarities between the contents of the post-pharyngeal gland and CHC profiles [13]. Taken together, these studies highlight only a small fraction of the complex pheromone biochemistry responsible for the organization and coordination of ant societies.
Over and above the role of CHCs, ants have been described as “walking chemical factories” because they rely on a large array of exocrine glands that collectively produce the semiochemical releasers for many complex social behaviors [1]. For example, in Formica argentea, undecane is produced in high concentrations in the Dufour’s gland, where it is likely to act as an alarm pheromone component [14]. In addition to its role in predation and defense, the poison gland of Formicidae produces formic acid, which may act synergistically with other compounds that elicit alarm responses in the Dufour’s gland [15,16]. Even more notorious is fire ant venom, which is comprised of hydrophobic dialkylpiperidines, known as solenopsins, used for predation and defense [17]. Moreover, even closely-related species may have strikingly different exocrine gland composition. This is illustrated in studies that examined the phylogenetic relationship of Camponotus floridanus to C. atriceps, one which was contested for a time with some suggesting that the two species were synonymous [18]. However, the ratio of compounds in the Dufour’s gland was observed to be notably different, with certain compounds, such as 2-methyldecane and heneicosane, present in only one species or the other [19]. This distinct phylogeny is also consistent with studies demonstrating that trail-following behaviors are evoked by distinct hindgut components found in each species. In this regard, C. floridanus is sensitive to nerolic acid, while C. atriceps relies on 3,5-dimethyl-6-(1’-methylpropyl)-tetrahydropyran-2-one [19]. In short, there is tremendous diversity of exocrine gland form and function among ants, including glands that may elicit behaviors that are unique to a given genera [1].
2. The Peripheral Olfactory System
The complex array of sensory neurons and support cells that together make up the peripheral olfactory system is the initial site of chemical detection and perhaps discrimination in ants. Here, pheromones, kairomones, and other semiochemicals are detected by an array of membrane-bound chemoreceptors expressed in suites of olfactory sensory neurons (OSNs). The function of these OSNs relies on a spectrum of signal transduction pathways that comprise both extra- and intracellular components centered around three classes of transmembrane chemoreceptors: ORs, gustatory receptors (GRs), and ionotropic receptors (IRs) (for a detailed discussion of these and other chemoreceptor proteins involved in Hymenopteran olfactory biology, we direct the reader to [5]). In brief, individual ORs are expressed in a subset of OSNs alongside the obligate and highly conserved odorant receptor co-receptor (Orco) [23]. The ORs are involved in the detection of pheromones and other general odorants which for ants notably include the CHCs [24,25]. ORs are hypothesized to derive from GRs, which are the most ancient chemoreceptor family in insects and, at least in Drosophila, are responsible for the direct (contact-based) detection of tastants as well as carbon dioxide [26]. Curiously, while empirical evidence suggests that ants are able to detect carbon dioxide [27,28,29], they have lost the canonical CO2 receptors found in dipteran species [21,30]. The IRs are derived from ancestral glutamate receptors and form an independent lineage of chemoreceptors that are, in Drosophila, responsible for the detection of acids and aldehydes [31,32,33]. Beyond these primary chemoreceptor families, there are a number of other ancillary support proteins involved in olfactory signaling. These include odorant degradation enzymes (ODEs) and a variety of odorant binding proteins (OBPs) and chemosensory proteins (CSPs). The former, as their name implies, facilitate the degradation of odorants [34,35], while the function of the latter is not as well understood. However, it is commonly thought that the OBPs and CSPs may facilitate odor transport through the sensilla lymph [36], although they are evidently not required for olfactory responsiveness [37,38].
The OSNs subtend hair-like sensilla that are stereotypically distributed along the antennae and other chemosensory appendages [39]. For ants, there are several different types of sensilla that vary in function, innervation, and morphology [40,41]. These notably include basiconic (broadly chemoreceptive including CHCs), ampullaceal (putatively CO2 receptive), chaetic (contact-based chemosensation), coelocapitular (hygro- and thermoreceptive), coeloconic (chemoreceptive), and trichoid (chemoreceptive) sensilla. In contrast to the relatively simple Dipteran olfactory system, which may have only a handful of OSNs in each sensillum, ant sensilla may contain over 130 OSNs [40]. In addition, there are important sexual dimorphisms with respect to the broad morphology of the antennae and the composition of sensilla between female and male ants. The basiconic sensilla, which presumably house the OSNs involved in CHC detection [42,43], are notably absent in males and likely reflect the distinct physiological function of behavior of the different members of the colony [40,44,45]. Altogether, the peripheral olfactory system in ants shares many features in common with other insect species; however, evolution has produced an unparalleled level of complexity in ants that is unrivaled even by their much more studied Dipteran counterparts (Figure 2).
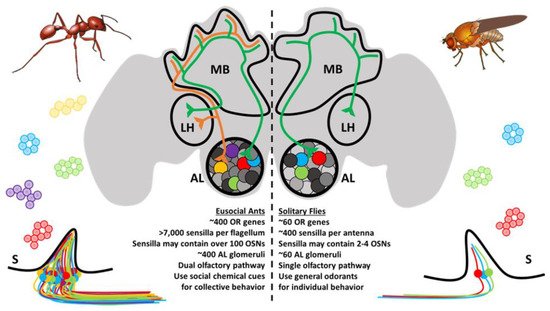
Figure 2. Ants and other eusocial Hymenopteran have a remarkably complex olfactory system relative to fruit flies and other solitary insects. Ant sensilla (S) may contain OSNs that are up to two orders of magnitude more numerous than those of the fruit fly. The antennal lobe (AL) glomeruli are also more numerous and follow a different developmental trajectory. Odor processing in higher-order brain structures, including the mushroom bodies (MB) and lateral horn (LH), occurs through a novel dual-olfactory processing pathway.
3. Central Olfactory System
3.1. An Overview of the Central Olfactory System in Insects
At a cellular level, the fundamental organization of the olfactory system is remarkably similar between vertebrate and insect species [58,59]. Across this broad evolutionary distance, diverse OSNs residing in an aqueous milieu receive chemical messages from the environment, and this information is relayed to the central brain via dedicated axonal tracts, converging on secondary neurons, local interneurons, and glial cells that together constitute the neuropil which forms the stereotypic glomeruli of the vertebrate olfactory bulb ortholog known in insects as the antennal lobe (AL) [60,61]. Until recently, it was doctrine that a single glomerulus was typically innervated by a specific corresponding set of peripheral OSNs, many of which express the same chemoreceptor [62,63]. There may, however, be important exceptions to this rule as emerging studies from Drosophila and the yellow fever mosquito Aedes aegypti reveal that a single OSN may co-express receptors from different chemoreceptor families and are linked to multiple AL glomeruli [64,65]. In any case, having arrived at their respective (or collective) AL glomeruli, synaptic connections relay information to a collection of secondary glomerular neurons, known in insects as AL projection neurons, which are comparable to vertebrate olfactory bulb mitral and tufted cells. The initial processing of peripheral olfactory information that eventually leads to odorant discrimination and presumably perception occurs through the combinatorial activation of glomeruli that is transformed through integrative (often inhibitory) crosstalk between glomeruli via local interneurons [66,67,68,69,70]. Projection neurons subsequently connect the olfactory bulb or AL to the olfactory cortex and other central brain structures in vertebrates or, in the case of insects, to the mushroom bodies and lateral horn of the protocerebrum [60,61]. In ants and other Hymenoptera, projection neurons are organized into a unique, dual olfactory pathway consisting of a medial and lateral output tract connecting to higher order brain structures which may improve olfactory information processing (Figure 2) [71,72]. These structures are then responsible for more complex cognitive processes. It has been suggested that insect mushroom bodies are responsible for learning and memory [73,74], whereas the lateral horn may play a role in learned and innate behavioral responses [75].
3.2. Structure and Function of the Antennal Lobe
While there are indeed many parallels between the insect and vertebrate olfactory systems, there are also notable differences in terms of scale, structure, and function. Mice have well over 1000 olfactory bulb glomeruli which, following the oft-cited, “one-receptor-one neuron-one glomerulus” rule [76,77], derives from a correspondingly similar number of ORs [78,79]. In contrast, Drosophila maintain 62 ORs and a comparable number of AL glomeruli [63,80]. As one might expect given their significantly larger OR repertoires, the complexity of ant ALs falls somewhere in between—the clonal raider ant Ooceraea biroi, for example, has approximately 500 glomeruli [41] whereas leaf-cutting Atta vollenweideri have about 390 among the smaller worker caste and 440 among the larger workers [81]. Importantly, the precise composition of the AL in ants and other eusocial Hymenopteran varies dramatically among different colony members within a given species with respect to age, task, and morphology [81,82,83,84,85]. Previous experience and exposure to different environmental conditions may also lead to changes in glomerular volume, odor coding, and behavior [86]. A distinct group of larger Camponotus workers (“majors”) have a correspondingly larger glomerular volume but fewer glomeruli than minor workers [85]. By contrast, larger workers in the leaf-cutting ant A. vollenweideri have a greater number of glomeruli than minor workers [81]. Interestingly, high volume macroglomeruli, which are about 9–10 times larger than average glomeruli, have also been identified in the larger worker caste of leaf-cutting ants, and these may be responsible for the detection of trail pheromones [87]. Furthermore, the ALs display profound sexual dimorphisms. In C. japonicas, sterile female workers and virgin queens have roughly 430 glomeruli, whereas the AL of males is reduced to only 215 glomeruli [88]. In C. japonicas, as well as other Hymenopteran species such as the honeybee Apis mellifera, males also have larger macroglomeruli structures, which are thought to be involved in the detection of sex pheromones [45,88,89,90,91]. These male-specific characteristics may reflect their marginalized role as short-lived reproductives. Overall, these changes likely reflect the unique behavioral and reproductive tasks carried out by different members of an ant colony.
The detection of social cues including pheromones and chemical blends such as CHCs distinguish ants and other eusocial species from solitary insects; however, it is noteworthy that odor coding in the AL is conserved across a broad evolutionary distance. Across insects and mammals, for example, the neuronal representation of general odorants among the AL glomeruli are organized and structured around distinguishing features of a given chemical class, including chain length and functional groups [92], and these activation patterns are consistent across members of a given species [72,93]. While glomerular activation patterns for both pheromones and non-pheromone odorants may overlap in ants [72,94], other studies have shown discrete clusters of glomeruli responsible for the detection of alarm and trail pheromone signals [87,95] and this is consistent with studies conducted in moths, which have dedicated macroglomerular complexes involved in the detection of sex pheromones [96] in addition to ordinary glomeruli that process both general plant volatiles and sex pheromones [97].
3.3. Olfactory Sensory Neurons and the Ontogeny of the Antennal Lobe
Another notable difference between the insect and vertebrate olfactory systems concerns the relationship between diverse sets of OSNs and the ontogeny of the AL glomeruli. In Drosophila, AL development occurs through three phases that begin at the start of pupation when dendrites from second-order projection neurons arrive at stereotypic sites in the brain [98]. In the second phase, OSN axons from peripheral olfactory appendages arrive at target sites in the proto-antennal lobe. This second phase notably occurs prior to OR gene expression and, not surprisingly, there are no significant structural alterations to the glomeruli of orco null mutant Drosophila [99]. Furthermore, OSNs survive through development but degenerate later in adulthood. This is in contrast to mice and other mammals, where functional ORs and OSNs are required for proper axon targeting [100] and are capable of regeneration [101]. In the final phase, fruit fly projection neurons and the axons from OSNs establish local synaptic connections to the exclusion of neighboring cells to create discrete glomeruli.
Arguably, the most compelling distinction about the olfactory system in ants compared to fruit flies and mosquitoes was the recent observation that Orco is required for the proper development of the AL glomeruli [102,103]. This rather unexpected difference in ant brain development was first described in two parallel reports using CRISPR-Cas9 gene editing to knock out Orco in the jumping ant Harpegnathos saltator [102] and the clonal raider O. biroi [103]. In addition to a profound loss of olfactory sensitivity, as well as the alteration of several behavioral phenotypes that were both anticipated, orco mutant ants displayed significant reductions in both OSN populations and the number and volume of AL glomeruli. More recently, AL development in O. biroi has been closely examined during the critical two-week pupation period [104]. In contrast with Drosophila [99], OR expression occurs much earlier in ant development, before the formation of glomeruli [104]. Indeed, Orco expression was high on the first day of pupal development, and almost all of the nearly 500 ant ORs were expressed by day 2 of the pupal stage. Moreover, while Orco is localized to the dendrites and cell bodies of fruit fly OSNs [99], in the clonal raider ant, it is also found in OSN axons and axon terminals in the brain. Here, unilateral antennal ablations (that impact only the ipsilateral half of the bilaterally symmetric ALs) on the first day of pupation resulted in significantly reduced glomeruli in adults. When antennae were ablated later in pupation, development was arrested, but any glomeruli that had already formed survived to adulthood. When antennae were ablated in adult callow workers, AL glomeruli remain for at least two weeks. Taken together, this suggested that orco mutants have impaired AL development due to loss of OSNs, which were necessary for the formation of glomeruli but not their maintenance. Curiously, approximately 90 glomeruli survived both the ablation treatment and in the orco null mutant [103,104]. The authors suggested these remaining glomeruli may be a more basic template upon which the remainder of the more complex AL forms.
Developing a topographical map of the AL in ants, as is being done in the honeybee [105], would catalyze the effort to provide a better understanding of odor coding in the glomeruli. These insights may shed light on the role of the mysterious 90 surviving glomeruli, if they have a function at all, and how their development may differ from the remainder of the AL. Ultimately, however, we are left with more questions than answers. This is especially so in light of recent studies in fruit flies and mosquitoes demonstrating that subsets of olfactory neurons co-express ORs, IRs, as well as potentially other receptor classes [64,65]. Such polymodal neurons display non-canonical relationships to the AL that upset the “one receptor-one neuron-one glomerulus rule” and provide a fruitful avenue for future research.
4. Genomics, Evolution, and the Regulation of Chemosensory Genes
Over the past decade, considerable progress has been made toward understanding olfactory genomics in eusocial insects [5]. During this time, more than 50 Hymenopteran genomes have come online, and sequencing efforts for many more are currently underway [106,107]. One of the most notable scientific discoveries resulting from this ever-growing repository of genomic data was the identification of significant changes in the chemoreceptor families [21,22,108,109,110,111,112,113,114]. Specifically, there has been a massive expansion of ORs through gene birth-and-death evolution across Apocrita that directly correlates to the degree of eusociality [21,22]. Among these, ants boast the largest number of ORs. Genome sequencing across the evolutionarily basal suborder Symphyta, which is devoid of any eusocial species, has been considerably more limited. One bioinformatics study completed thus far in Symphyta has revealed that the genome of the solitary wheat stem sawfly Cephus cinctus has not undergone the same expansion of ORs as seen in Apocrita [115]. A notable exception to the eusocial-driven expansion of OR gene families is the genomes of several species of solitary wasps, including Nasonia vitripennis and Microplitis demolitor, each of which have more ORs than that of the eusocial honeybee A. mellifera [22,116]. Looking at chemoreceptors beyond ORs, the genome of the dampwood termite Zootermopsis nevadensis has a greatly expanded family of IRs [117]. Similarly, cockroaches have the largest number of chemoreceptors of any insect species described to date, with massive expansions of the IR and GR families [118]. While insufficient to fully explain the macroevolution of eusociality, the expanded capacity to detect and communicate chemical information likely facilitated the acquisition of the broad range of social behaviors that doubtlessly also provided an adaptive advantage across diverse environments. These early genomic studies in Hymenoptera provided a clear sense of direction for future research spurred by the concurrent development of molecular tools in eusocial insects.
This entry is adapted from the peer-reviewed paper 10.3390/insects12030252
