Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Mitochondria-associated membranes (MAMs) play a vital role in the complex crosstalk between the ER and mitochondria. MAMs are known to play an important role in lipid synthesis, the regulation of Ca2+ homeostasis, the coordination of ER-mitochondrial function, and the transduction of death signals between the ER and the mitochondria.
- endoplasmic reticulum
- mitochondria
- MAMs
- Ca2+
- apoptosis
1. Introduction
Apoptosis is a programmed form of cell death and can occur via three main pathways. The first pathway is the intrinsic apoptosis pathway; initiated by a range of factors, including the effects of growth factors or hormones, radiation, or cytotoxins [1]. This process involves the enhancement of pro-apoptotic signals and the weakening of anti-apoptotic signals. An imbalance in the regulation of apoptosis ultimately leads to changes in the permeability of the mitochondrial outer membrane and the release of pro-apoptotic substances from the mitochondria. These pro-apoptotic substances promote apoptosis by activating the apoptotic executive protein caspase-9, inhibiting IAPs or via the direct cleavage of DNA [2,3,4,5,6,7]. The second pathway is the extrinsic apoptosis pathway in which apoptosis is activated by the binding of specific ligands such as FasL to transmembrane receptors which contain “death domain” such as FasR. Activated death receptors then recruit adaptor proteins in the cytoplasm to assemble an apoptosis-inducing signal complex, which then activates caspase-8 to initiate apoptosis [8]. Caspase-8 can also cleave Bid to initiate the intrinsic apoptotic pathway, which plays an important role in the process of apoptotic signal amplification [9,10]. The third pathway is the perforin/granzyme pathway in which cytotoxic T cells or NK cells induce target cell apoptosis by secreting granules containing perforin or granzymes [11].
Moreover, the Bcl2 family plays a vital role in cell apoptosis. The Bcl2 family consists of 25 members, and they can be divided into pro-apoptotic proteins (Bax, Bak, etc.) and anti-apoptotic proteins (Bcl2, Bcl-XL, Mcl-1, etc.) according to their different functions. Furthermore, pro-apoptotic proteins can be divided into pro-apoptotic proteins with multiple domains and BH3-only proteins, and BH3-only proteins also can be divided into “activator” (Bim, tBid, etc.) and “sensitizer” (Bad, Bik, etc.) based on their specific mechanism of action [12]. The activated pro-apoptotic members can assemble on the outer mitochondrial membrane and change the permeability of the outer mitochondrial membrane, and promote the release of cytochrome c, AIF, Smac/Diablo and other apoptosis-inducing factors from the mitochondria [13]. Anti-apoptotic members mainly antagonize the effects of pro-apoptotic ones through protein–protein interactions to maintain the integrity of the mitochondrial outer membrane. The “activator” BH3-only members can directly activate the pro-apoptotic proteins and promote the occurrence of apoptosis, while the “sensitizer” BH3-only members can interact with the anti-apoptotic in a protein–protein interaction way to relieve the effect of the pro-apoptotic proteins [14].
As it is mentioned above, the mitochondria play a central role in the cell apoptosis, and the crosstalk between mitochondria and other organelles may impact the apoptosis process. Recent studies revealed that the communication between ER and mitochondria can influence the cell apoptosis, thus affecting the cell fate.
The ER is a key organelle that plays a crucial role in Ca2+ storage, lipid synthesis, protein folding, and assembly [15,16,17,18]. Mitochondria are the “energy factories” of eukaryotic cells, and provide energy to drive the physiological processes of cells; they also play a key role in the process of apoptosis [19,20,21]. The ER and mitochondria are independent of each other but are also closely associated in structure and function. The first spatial connection between the ER and the mitochondria was reported in the 1950s following a study of hepatocytes by transmission electron microscopy [22,23]. In 2006, an electron tomography study further confirmed the complex relationship between the ER and the mitochondria [24]. It is now believed that the mitochondrial surface juxtaposed to the ER in mammalian cells is up to 5–20% due to different cell types [25,26]. Based on this close structural connection, the ER is able to respond to a variety of stress stimuli and can transmit these stress signals to the mitochondria [27,28], thereby initiating the mitochondrial stress response. Similarly, the mitochondria can transmit signals to the ER, thus ensuring the efficient execution of compensatory responses or cell death events. Due to the special function of these precise structural associations, this biological system is usually investigated as a relatively independent sub-organelle structure referred to as a “mitochondrial-related membrane structure.”
2. The Structural Characteristics of MAMs
The structure of MAMs does not remain constant; rather, the structure of MAMs changes dynamically in response to the cell status. The width of the gap between the ER and the outer mitochondrial membrane varies from 10 to 100 nm [29,30]; the width of this gap is usually 10–15 nm at the smooth endoplasmic reticulum and 20–30 nm at the rough endoplasmic reticulum; these spatial differences may be related to the presence of ribosomes [7,31]. Different proteomic analysis of the structure of MAMs has revealed 991 [32] and 1212 [33] different proteins in MAMs [34]. Mass spectrometry analysis divided these constituent proteins into three categories: Proteins that are specifically present in MAMs; proteins that exist simultaneously in MAMs and other organelle structures; and proteins that only exist temporarily in MAMs [33]. These proteins are involved in a wide range of processes, such as structural maintenance, lipid synthesis, the regulation of Ca2+ homeostasis, mitochondrial dynamics, and apoptosis.
3. The Structure Maintenance of MAMs
There are thousands of proteins in MAMs; the roles of these proteins are known to vary widely. Some of these proteins play a tethering role in the maintenance of MAMs [31]. According to our understanding, tethering proteins should exhibit certain characteristics. For example, tethering proteins could be (1) proteins or protein complexes that directly participate in the physical connection between the ER and the mitochondria, or (2) interfering proteins or protein complexes that can directly cause changes in the width of gap, the area of contact, or the number of contact sites between the ER and the mitochondria. These proteins and protein complexes are introduced below (Figure 1).
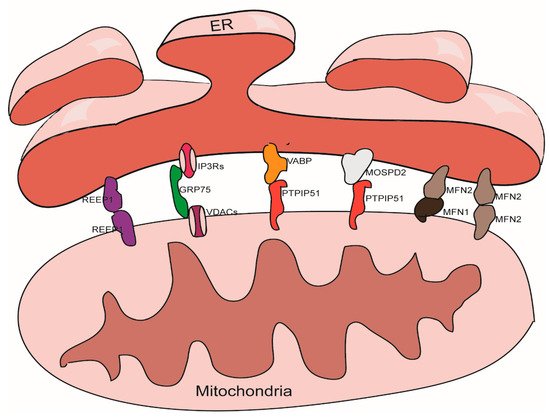
Figure 1. Tethering proteins that participate in the mitochondria-associated membranes (MAMs) structure maintenance.
3.1. The IP3Rs-Grp75-VDACs Complex
IP3Rs are important Ca2+ outflow channels on the surface of the ER and mediate the release of Ca2+ from the cavity of the ER to the cytoplasm [35,36]. VDACs are ions channel located on the outer membrane of the mitochondria; these mediate the movement of a variety of ions and metabolites in and out of mitochondria, and participate in a range of cellular activities, including apoptosis, metabolism, and the regulation of Ca2+ [37]. IP3Rs and VDACs are connected by Grp75 to maintain the structure of MAMs [38]. The overexpression of VDACs is known to enhance the connection between the ER and the mitochondria and thus improve Ca2+ flux from the ER to the mitochondria [39]; while silencing VDAC1 exhibits a reduction in the connection between Grp75 and IP3R1 indicating a reduction of ER-mitochondria interactions [40]. Cells overexpressing Grp75 showed higher number of IP3R1–VDAC1 interaction sites [41]. Silencing IP3R1 or Grp75 can also reduce the connection between VDAC1 and Grp75 or IP3R1 [42].
3.2. The VAPB-PTPIP51 Complex
VAPB is located in the membrane of the ER and participates to the activation of the IRE1/XBP1 axis in the ER unfolded protein response [43,44]. VAPB can form a complex with the outer mitochondrial membrane protein PTPIP51 and help to maintain the structure of MAMs. A mutant form of VAPB, VAPBP56S, exhibits a stronger affinity for PTPIP51, thereby promoting the transfer of Ca2+ from the ER to the mitochondria; knocking out either of these two genes can reduce the transfer of Ca2+ signals [45]. Other studies have shown that knocking down either of these two proteins will reduce the level of contacts between the ER and the mitochondria [46,47].
3.3. The Mfn1/Mfn2 Complex
In addition to being located in the outer mitochondria membrane and participating to the mitochondrial fusion [48], Mfn2 can also localize on the surface of the ER. Mfn2 participates in the structural maintenance of MAMs by forming homodimers or heterodimers with Mfn1/2 on the outer membrane of the mitochondria. The function of the Mfn1/Mfn2 complex with regards to maintaining the structure of the MAMs was first discovered in 2008 [49]; this role has also been confirmed by several other studies [50,51]. However, some studies have yielded contradictory results [52,53], it is now well established that Mfn2 plays a role in the endoplasmic reticulum stress (ERS) response; the ERS induced by knockdown of Mfn2 can tighten the association between the ER and the mitochondria [54].
3.4. The MOSPD2-PTPIP51 Complex
MOSPD2, another member of VAP family, a protein that locates on the surface of the ER membrane, plays a role in connecting the ER with other membrane structures. It can also bind with proteins containing a small VAP-interacting motif, named FFAT [two phenylalanines (FF) in an acidic track (AT)] via an MSP (Major Sperm Protein domain), such as PTPIP51 on the outer membrane of the mitochondria [55].
3.5. REEP1
REEP1 is a protein that is located in the outer membrane of the ER and the mitochondria. REEP1 helps regulate the morphology of the ER. Studies have shown that REEP1 directly connects the ER and the mitochondria through oligomerization and participates in forming the structure of MAMs. In addition, through bending ER membranes, REEP1 makes it topologically possible for the ER to wrap around the mitochondria, which helps to form MAMs [56].
3.6. Other Proteins Involved in MAMs Maintance
In addition to these tethering proteins, there are some proteins that do not directly participate in the structural maintenance of MAMs. However, these proteins do affect the structure of MAMs via protein–protein interactions (Table 1). In addition to being present in the cytoplasm, α-Synuclein can also be incorporated in MAMs [57]. α-Synuclein can promote the Ca2+ transfer from ER to mitochondria by increasing the ER and mitochondria contacts; and further study showed that the C-terminal of α-Synuclein is essential to tighten the contacts [58]. Some studies revealed that the α-Synuclein existing in MAMs results in the dis-regulation of Ca2+ and lipid metabolism, which promotes substantia nigra pars compacta neurons to die, leading to the progression of PD [59]. In addition to playing an anti-apoptotic role in cells and participating in mitochondrial dynamics, DJ-1 can still exist in the MAMs, thus enhancing the connection between the ER and the mitochondria and the crosstalk between the two organelles; this effect may be related to P53 to some extent (an antagonistic relationship) [60]. Existing studies suggest that DJ-1 can bind directly to the IP3R-Grp75-VDAC complex and affect its stability. The knockout of DJ-1 resulted in the aggregation of IP3R3 in MAMs and a reduction in the formation of the IP3Rs-Grp75-VDACs complex; it is possible that this is related to the pathophysiological process of obesity [61]. Although the precise mechanism remains obscure, it has been ascertained that DJ-1 can affect the structural stability of MAMs. This also implies that MAMs may play a role in the pathogenesis of Parkinson’s syndrome. TDP-43 and FUS are proteins that are related to ALS/FTD and can activate GSK-3b by down-regulating the phosphorylation levels of serine 9 by GSK-3b. Once activated, GSK-3b can reduce the connections between VABP and PTPIP51, thereby detaching the ER from the mitochondria [47,62]. PDK4 can directly interact with the IP3Rs-Grp75-VDACs complex in MAMs and may promote the formation of this complex by regulating phosphorylation, thus increasing the area of contacts between the ER and the mitochondria [42]. In addition to participating in the post-transcriptional modification of proteins, TG2 can also be incorporated in MAMs and act directly on Grp75 to increase the number of ER-mitochondrial contacts and thus participate in the structural maintenance of MAMs [63]. The precise function of TpMs (a type of keratin binding protein that is partly located in the mitochondria) remains unclear although data indicates that this protein can negatively regulate the ER-mitochondria connections in a Mfn2-dependent manner [64]. It is generally believed that CypD, a protein located in the mitochondrial matrix, can also be incorporated in MAMs, and directly act with the IP3Rs-Grp75-VDACs complex to regulate the stability of this complex. Inhibiting the function of CypD can down-regulate the binding of Grp75 with IP3Rs and VDACs, affecting the transfer of Ca2+ between the two organelles [65]. FUNDC1 is known for maintaining the stability of IP3R2 in MAMs by direct binding, and it enhances the level of contacts and the communication of Ca2+ between the ER and the mitochondria [66]. Presenilin-2 can also promote the connection and the transfer of Ca2+ signals between the ER and the mitochondria in the presence of Mfn-2; these findings were confirmed by overexpression and knockdown experiments, which suggested that presenilin-2 works with the Mfn1/Mfn2 complex [67]. FATE1 can reduce the level of contacts between the ER and the mitochondria and downregulate the transfer of Ca2+ with an impaired sensitivity to Ca2+-related apoptosis [68]. In addition to participating in the morphological regulation of ER, NogoB can increase the gap width of MAMs and affect their function [69]. PERK, which plays an important role in ERS, can increase the level of connectivity between the ER and the mitochondria by interacting with Mfn2, and thus promote the transduction of ERS signals to the mitochondria [70,71]. Although these proteins are not considered to be directly involved in maintaining the structure of MAMs, they still attract research attention due to their specific regulatory effects on the structure of MAMs and their involvement in the pathological processes underlying many neurodegenerative diseases.
This entry is adapted from the peer-reviewed paper 10.3390/cells10030657
This entry is offline, you can click here to edit this entry!
