Surface layers (S-layers) are the most common outermost cell envelope components of prokaryotic organisms (bacteria and archaea). The lattice formed by S-layer proteins are highly porous structures with identical pores in the nm-range. This feature can be utilized to fabricate ultrafiltration membranes with a very sharp specific molecular weight cut off. Moreover, S-layer lattices reveal an intrinsic antifouling characteristics, which results in a negligible clogging of the filter.
- S-layer protein
- ultrafiltration membrane
- biomimetic
- nanotechnology
- antifouling
- bioinspired filter membrane
1. Introduction
Ultrafiltration (UF) is a membrane-based filtration, in which pressure or concentration gradients induce a separation through a semipermeable membrane. UF membranes processes are used in industry and research for purifying and concentrating suspended solids, macromolecules and colloidal particles, particularly protein solutions of 2 to 100 nm in diameter corresponding to a molecular weight of 103 to 106 Dalton (Da) [1,2,3]. UF membranes with a pore size ranging from 2 to 100 nm are defined by the specific molecular weight cut off (MWCO). The latter refers to the lowest molecular weight solute (in Da) in which 90% of the solute is retained by the membrane.
Beside the implementation of UF in research, UF membranes are an established separation technology utilized in many industrial process applications worldwide. UF membranes have the unique ability to purify, concentrate, and fractionate of a large range of macromolecules and proteins (e.g., milk proteins and enzymes, silt, plastics, oil, silica, nanoparticles, endotoxins, and viruses) via a physical membrane barrier determined by the MWCO. Thereby the UF membranes ensure a very consistent rejection and flux performance [2]. Industrial fields of application range from dairy, biotechnology and pharmaceutical use over food, beverage, and plant extracts to wastewater treatment [1,2,3].
Most UF membranes comprise of polymer materials like polysulfone, polypropylene, cellulose acetate, and polylactic acid [1,3] and have a surface porosity which is usually lower than 10%. They also show a size distribution of the pores varying by up to an order of magnitude [4]. Due to the presence of differently sized pores, the flux is strongly biased to the larger ones, with typically 50% of the solvent passing through 20 to 25% of the pores [4]. This leads to a heterogeneous flow pattern normal to the membrane surface. The heterogeneous porosity of UF membranes makes them susceptible to flux decline by loss of the larger pores [2,4] and thus, not well suited for the fractionation of close-sized macro-solutions.
In the present entry we will introduce the highly porous crystalline monomolecular protein lattices, which represent the outermost surface layer (S-layer) in many bacteria and almost all archaea (Figure 1) as the MWCO-determining functional layer [5,6,7,8,9,10,11,12,13]. The S-layer ultrafiltration membrane (SUM) comprises of a microfiltration membrane onto which S-layer-carrying cell wall fragments or S-layer protein (SLP) self-assembly products are deposited in a pressure-dependent manner [6,8,9,14,15,16,17,18,19]. Whereas the active ultrafiltration layers of most synthetic membranes show a porosity of up to 10%, the crystalline S-layers reveal a porosity of up to 70% (Figure 2). The preformed SUM can, if desired, be chemically crosslinked to enhance the mechanical and thermal robustness. In the following we will report on the intrinsic features of bacterial SLPs, their molecular sieve property and how SUM are manufactured and modified to show optimal performance in filtration processes. Moreover, SUMs may also be utilized as binding matrices for the formation of complex composite structures for various biotechnological and pharmaceutical applications.
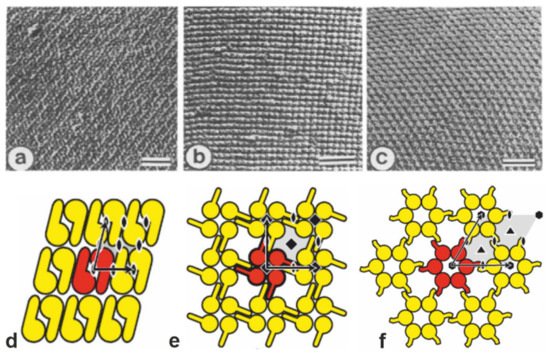

2. Ultrastructure and Self-Assembly of S-Layer Proteins
3. S-Layers as Molecular Sieves
4. S-Layer Ultrafiltration Membrane (SUM)
SUMs are made by depositing either sheet like S-layer self-assembly products (see Section 2) or S-layer carrying cell wall fragments (approx. 0.5 to 1.5 µm size) on commercial microfiltration membranes with a spongy structure or radiation-track type membranes having pore sizes of 0.05 to 0.1 µm in a pressure dependent procedure (Figure 3) [10,11,59,60]. S-layer carrying cell wall fragments were prepared from whole cells by extracting the cytoplasmic membrane with Triton X-100 under conditions, which preserved the integrity of the S layer and the peptidoglycan-containing layer [53]. Like tiling a roof, this procedure generates a coherent coating, composed of multilayers of S-layer fragments arranged in random orientation. Interestingly, the pores of the constituent (monomolecular) S-layer lattice solely determined the MWCO of these composite multilayered structures.
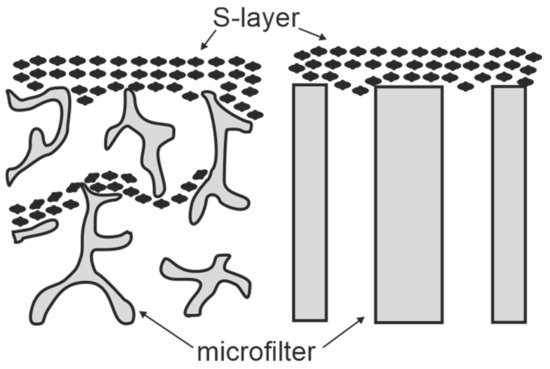
After deposition, the SLP lattices are inter- and intramolecular crosslinked with glutaraldehyde and Schiff bases are reduced with sodium borohydride [10]. The nominal MWCO of SUMs from S-layers of mesophilic and thermophilic Bacillaceae revealed no significant difference and was in the range of 30.000 to 40.000 Da (Figure 4) [10,12,53]. As estimated by TEM and AFM, the porosity of the SLP lattice lies between ca. 30 and 70% and is therefore significantly higher than that determined for polymeric UF membranes with a maximum of 10%.

Native S-layers were found to be zwitterionic, thus preventing nonspecific adsorption of charged macromolecules, which would lead to pore blocking (fouling) [61]. After crosslinking the S-layer protein lattice with glutaraldehyde, which involves loss of positively-charged amino groups, SUMs revealed a net negative charge on the surface and inside the pores [10,17,19,62]. SUMs possessing a high density of free carboxylic acid groups did not adsorb negatively charged macromolecules but strongly interacted with proteins exhibiting a positive net charge [14,41,56]. In ultrafiltration processes, protein adsorption, which is considered as the first step in membrane fouling is expressed in terms of flux losses for particle free water after protein filtration [63,64]. By using SUMs composed of two-dimensional crystalline protein lattices with defined pore sizes and defined surface charge, it was possible for the first time to determine correlations between the pore size and the net charge of the active filtration layer, the molecular characteristics (dimensions, net charge) of adsorbed protein molecules and the flux losses caused by adsorption [19,62,65]. The flux of SUMs produced with S-layers from Geobacillus strains ranged from 150 to 250 L m−2 h−1 when measured at 0.2 MPa with water [12]. Although the initial flux of SUMS correlated with the number of S-layer fragments deposited on the microfiltration membrane, the dimension of the active filtration layer did not influence the MWCO [10,59,60]. As S-layers are periodic structures, they exhibit repetitive identical physicochemical properties down to the sub-nanometer scale not only on the surface but also in the pore area. Thus, surface properties and molecular sieving as well as antifouling characteristics of SUMs could be tuned by chemical modifications involving activation of carboxylic acid groups with carbodiimides and subsequently converting them with differently sized and charged nucleophiles [41,42,62,66]. Thereby, SUMs can be manufactured with different net charge, hydrophilic or hydrophobic surface properties and separation characteristics [62]. A correlation was observed between the molecular size of attached nucleophiles and the shift of the sharp rejection curve to the lower molecular weight range [17,62]. This confirmed that a pore size reduction had occurred due to an aperture like enlargement of modified carboxylic acid groups exposed in precise position and orientation on protein domains inside the pores. It was also demonstrated that both, the net charge of the S-layer lattice and that of the protein molecules used in filtration experiments determine the solute rejection characteristics of SUMs [42,62,67]. Thus, reams of chemical and/or genetically induced modifications allow the adaptation of SUMs to very specific process requirements. In comparison with conventional ultrafiltration membranes produced by amorphous polymers, SUMs exhibited an extremely low unspecific protein adsorption (membrane fouling) in buffer solutions. Concerning the chemical stability, SUMs composed of inter- and intramolecular crosslinked S-layer lattices were resistant towards many organic solvents (ketones, alcohols, chlorinated hydrocarbons, aromatic compounds), chaotropic agents, acidic and alkaline pH conditions (pH 1–13 at 20 °C for 160 h), and shear forces. These studies demonstrated that SUMs are equivalent in their chemical resistance to polyamide membranes [10,14,18,66,68].
This entry is adapted from the peer-reviewed paper 10.3390/membranes11040275
