The family of coronaviruses (CoVs) uses the autophagy machinery of host cells to promote their growth and replication. Pharmacological or pharmacogenomics tools might be used to modulate autofaphy, and these processes stand out potential targets to combat COVID-19.
- COVID-19
- autophagy
- pharmacology
1. Introduction
On 2 September 2020, WHO recommended corticosteroids as an effective treatment for seriously ill COVID-19 patients. Several other drugs were clinically used in the same effort to contain the deaths caused by COVID-19. Only in December 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom and the Food and Drug Administration (FDA) in the United States of America (USA) authorized the emergency use of Pfizer/BioNTech’s and Moderna’s vaccines against COVID-19 [5]. Nevertheless, worldwide vaccine plans are yet to be implemented and novel mutations of the SARS-CoV-2 are rapidly emerging [6,7] demanding continuous research on therapeutics to manage COVID-19.
2. Coronavirus Hijack the Autophagy Machinery to Foster Replication
Autophagy is triggered by the inhibition of mammalian target of rapamycin complex 1 (mTORC1), the primary regulator of nutrient signaling. Moreover, the mTORC1 complex is modulated by upstream regulators that transduce growth factors and energy signals. Autophagosome formation and its self-assembly are coordinated by enzymes and proteins located in the ER, such as phosphatidylinositol 3-phosphate (PI3P) and B-cell lymphoma 2 (BCL-2) interacting proteins Beclin-1/vacuolar protein sorting 34 (Beclin-1/Vps34) complex [40,41]. In addition to these proteins, the activating molecule in Beclin-1-regulated autophagy (Ambra1) plays a vital role as a regulator of autophagy, binding to Beclin-1, promoting the autophagosome formation [42]. Components of the autophagy machinery also participate in the secretion of invading pathogens. The use of autophagic machinery by CoVs was demonstrated, where the initiation of vesicle formation was inhibited by knocking out autophagy-related gene 5 (ATG5) or by wortmannin, suggesting that nsp6-induced autophagy was dependent on Atg5 and PI3K. Finally, transfecting the SARS-CoV open reading frame -8b and -3a into 293T and HeLa cells triggers lysosomal damage and ER stress, consequently inducing the translocation of Transcription Factor EB (TFEB) to the nucleus, a master regulator of lysosomal biogenesis and favoring the transcription of autophagy- and lysosome-related genes [54,55]. Defects in the molecular machinery for macroautophagy, such as the genetic inhibition of ATG5 or beclin-1 (BECN1) genes, consequently make mice and primary human astrocytes more susceptible to viral infections [56,57,58].
Conversely, other studies have highlighted the inhibitory effects of CoV nonstructural proteins on autophagy flux. In fact, overexpressing CoVs membrane-associated papain-like protease PLP2 (PLP2-TM) resulted in inhibition of autophagosome–lysosome fusion and blockade of autophagic flux in HEK293T, HeLa and MCF-7 cells [52]. Likewise, recent evidence described that Vero B4 cells infected with MERS-CoV exhibited reduced Beclin-1 levels, enhanced K48-polyubiquitylation of Beclin-1, reduced Atg14 oligomerization and blocked autophagosome-lysosome fusion [60]. Correspondingly, temporal kinome analysis of Huh7 and MRC5 cells infected with MERS-CoV displayed upregulated PI3K/AKT/mTOR and extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK)-mediated signaling [61].
An in-depth analysis of autophagy signaling, and metabolomics corroborated the notion that CoVs modulate PI3K/AKT/mTOR and AMPK signaling, showing that SARS-CoV-2 reduced glycolysis and protein translation by limiting the activation of mTORC1 and AMPK. It was shown that SARS-CoV-2 infection also downregulated spermidine and facilitated AKT1/S-phase kinase-associated protein 2 (SKP2)-dependent degradation of Beclin-1 [72].
Since autophagy may be one of the molecular mechanisms that allow cell invasion and virus replication, it is possible that some mutations may alter the autophagic process [78,79]. SARS-CoV-2, like any type of virus, accumulates mutations over time, and most of these mutations do not implicate in biological effects. However, some key mutations can alter viral biology to the extent of causing changes in its transmission and infection capacity [80]. Briefly, Table 1 summarizes the molecular machinery recruited in autophagy initiation and Figure 1 shows that autophagy mechanisms represent potential targets for pharmacological inhibition of CoVs infection and replication.
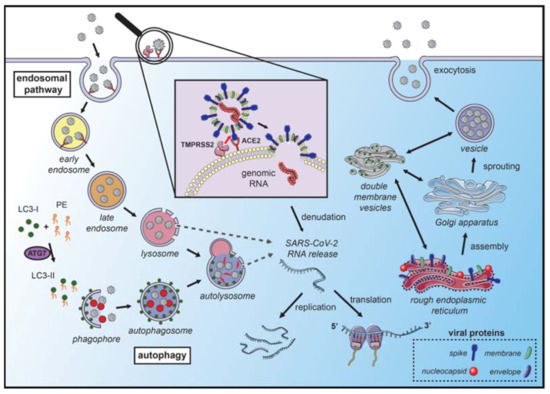
Figure 1. Coronavirus hijacks autophagy machinery to promote their replication. SARS-CoVs bind to the angiotensin-converting enzyme 2 (ACE2) receptor on the membrane surface and enter the host cell. The fusion with the membrane and the release of the genomic RNA into the cytoplasm occurs after the cleavage of the spike (S) protein, which can occur in several locations. S protein cleavage occurs on the cell membrane surface by the transmembrane protease serine 2 (TMPRSS2), which is associated with the ACE2 receptor, or by cathepsin-L and cysteine proteases in the endosomal system. The acidic pH in the lysosomes is necessary for the activity of cathepsin-L and S protein cleavage. Next, the endosomal cargo converges with the autophagic vacuoles in the lysosomes. Coronavirus nonstructural proteins colocalize with microtubule-associated proteins 1A/1B light chain 3A (LC3-II) in the endomembrane system, suggesting that autophagy plays a role in amplifying coronavirus replication. After fusion with the membrane, the genomic RNA is released and stripped of the nucleocapsid protein. Viral proteins are translated in the endoplasmic reticulum, which promotes the rearrangement of endoplasmic reticulum membranes and the formation of double-membrane vesicles, which are also localized with LC3 and autophagy-related proteins. The newly synthesized genomic RNA is then assembled into virions in intermediate compartments located between the endoplasmic reticulum and the Golgi apparatus and moves through the secretory pathway of the host and eventually released by exocytosis (the illustration was produced using the smart servier medical art vectors for publications and presentations licensed under the Creative Commons (CC BY 3.0)) [93].
3. Autophagy-Related Therapeutic Targets for COVID-19 Management
3.1. Lysosomotropic Agents
Chloroquine (CQ) and hydroxychloroquine (HCQ) are weak diprotic bases used as antimalarial drugs (Figure 2). These compounds accumulate in the endosome–lysosomal network of cells and neutralize the acidic pH, with consequent blockage of cathepsin activity and lysosomal fusion [104,105]. Previous studies showed that CQ displays a wide-spectrum of antiviral effects against CoVs, chronic HIV, and influenza viruses type A and B, both in vitro and in vivo [106,107].
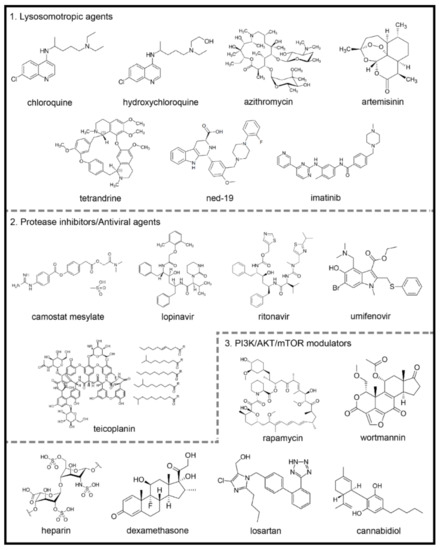
Figure 2. Chemical structures of potential autophagy-related drugs for SARS-CoV-2 infection. The drugs were divided in three groups according to their effects on the autophagy signaling pathway and possible effect against SARS-CoV-2 infection. The lysosomotropic agents (1) can prevent coronavirus infection by alkalinizing the acid pH in the endolysosomal system; some examples are chloroquine, hydroxychloroquine, azithromycin, artemisinin, two-pore channel antagonists (such as tetrandrine and ned-19) and imatinib. The protease inhibitors/antiviral agents (2) can inhibit the proteolytic cleavage of the spike coronavirus protein, which is necessary for viral entry into host cells; some examples are camostat mesylate, lopinavir, ritonavir, umifenovir and teicoplanin. The third group is composed by PI3K/AKT/mTOR signaling pathways modulators (3), which can modulate intracellular pathways related to autophagy and coronavirus infection; some examples are the rapamycin, wortmannin, the anticoagulant heparin, the glucocorticoid dexamethasone, losartan and cannabidiol. The figures for each chemical structure are from according to Wikimedia Commons (Public Domain).
Azithromycin is a broad-spectrum macrolide antibiotic that binds to the S50 ribosomal subunit of bacteria inhibiting its protein synthesis [121] (Figure 2). The antiviral efficacy of azithromycin has been demonstrated in different viral infections [122,123,124,125].
Artemisinin is isolated from the herb Artemisia annua L. [135]. The derivative compounds are sesquiterpene lactones with a unique endoperoxide bridge moiety primarily responsible for their biological actions [136] (Figure 2). ArtemiC, a micellar formulation of artemisinin, curcumin, frankincense (Boswellia) and vitamin C, which is administered by spraying, is in phase II of clinical trials for patients diagnosed with COVID-19 (NIH-Clinical Trials Database; Identifier: NCT04382040 and NCT04553705) [119].
Nicotinic acid adenine dinucleotide phosphate (NAADP) is an intracellular messenger that plays a vital role in the mobilization of Ca2+ in mammalians cells [145,146,147,148] by binding to two-pore channels (TPCs) [149,150]. Furthermore, NAADP has been reported as a potent Ca2+ mobilizing messenger and inducer of autophagy [151,152,153,154,155,156]. On the other hand, the TPC antagonists ned-19 and tetrandine (Figure 2) were postulated as possible blockers of lysosomal function, causing a further inhibition of autophagy on the degradation step [152].
Imatinib, a tyrosine kinase inhibitor developed in 2001, revolutionized the treatment of chronic myeloid leukemia [161], since its activity against the breakpoint cluster region gene-Abelson proto-oncogene (BCR-ABL) in cancerous cells [162] (Figure 2).
3.2. Protease Inhibitors/Antiviral Agents: The Prevention of Infection
1. Camostat Mesylate
Camostat mesylate inhibits the serine protease TMPRSS2 and prevents the entry of SARS-CoV-2 into the host cells [101] (Figure 2). Other proteases, including cathepsin-L, thermolysins, plasmins and trypsin, can act as a cofactor for virus entry into the host cell [173].
Lopinavir (ABT-378) is a potent protease inhibitor used to prevent HIV replication and spread [174] (Figure 2). It has been suggested that since SARS-CoV-2 contains structural components that are similar to other viruses, including HIV, it is plausible that this antiviral therapy could be used to treat patients with COVID-19 [176].
Umifenovir is currently used in Russia and China as a prophylaxis for the treatment of pulmonary infections caused by human influenza A and B viruses and HCV [179,180] (Figure 2). The proposed mode of action of umifenovir involves intercalation with membrane lipids, inhibiting viral fusion with the plasma membrane of the host cell. It has also been shown that the drug can bind to the membrane-bound clathrin protein and prevent endocytosis of the virus [179].
Teicoplanin is a clinically approved glycopeptide antibiotic that inhibits cathepsin L activity and blocks MERS-CoV and SARS-CoV entry into cells [184] (Figure 2).
3.3. PI3K/AKT/mTOR Modulators
Rapamycin is a PI3K/AKT/mTOR inhibitor and clinically proven macrolide that exhibits potent antitumor and immunosuppressive activity [103,186] (Figure 2). While the antiviral activity of rapamycin is controversial [103], it was capable of reducing porcine epidemic diarrhea virus [187], transmissible gastroenteritis virus (TGEV) and CoVs infectivity [188].
Heparin exhibited several antiviral actions [191,192,193,194], probably due to its structural similarity to heparan sulfate [195], a glycosaminoglycan formed by proteoglycans present on the surface of cells that participates in viral entry into eukaryotic cells as an initial anchoring domain [191,196] (Figure 2). Thus, heparan sulfate appears to modulate the entry of SARS-CoV into cells.
Glucocorticoids (GCs) are steroid hormones with potent anti-inflammatory and immunosuppressive actions used in the treatment of chronic inflammatory, autoimmune and allergic diseases [198,199] (Figure 2). During the SARS-CoV and MERS-CoV epidemics, GCs were widely used to decrease the exacerbated immune response caused by the uncontrolled release of proinflammatory cytokines observed during severe lung inflammation [200,201].
Several studies have shown that renin-angiotensin system (RAS) deregulation may be responsible for acute respiratory distress syndrome, which can be triggered by viruses (SARS-CoV, H5N1 and H7N9), bacteria and particles and molecules [232]. Therefore, excess angiotensin II may be primarily responsible for increased SARS-CoV pathogenesis [233]. Thus, these studies suggest that decreasing the angiotensin II levels or blocking the RAS pathway might attenuate acute lung injury severity.
It is known that SARS-CoV-2 infection leads to a proinflammatory cytokine storm [250]; thus, cannabidiol might decrease the levels of these cytokines and benefit patients infected with SARS-CoV-2 [251].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22084067
