Molecularly defined subunit vaccines are generally safer but often lack immunogenicity, due to the absence of key additional structural components needed for efficient activation of innate immunity. Such vaccines require the coadministration of an adjuvant [
96]. Few adjuvants are already licensed as part of vaccine formulations and some others are being used in the clinic [
97]. However, the development of adjuvants has been traditionally an empirical process [
98] as their molecular mechanisms of adjuvant activity are not fully understood [
95,
99,
100], hindering the rational design of improved, less toxic adjuvants for optimal matching with selected vaccine antigens [
101]. For long the immunological function of vaccine adjuvants has been subject of speculation [
95].
It is not straightforward to translate the newly discovered mechanisms of adjuvant activity to generally applicable approaches for rationally designed vaccines. Thus, a thorough knowledge of adjuvants and their immunological effects is needed to realize the full potential of rational vaccine design. [
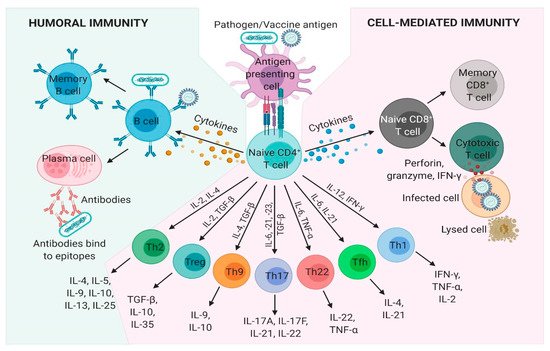
102]. These critical vaccine elements come in many forms and serve to initiate, accelerate, amplify, improve and prolong the immunological responses to antigens. Vaccine adjuvants have been classified in vaccine antigen delivery systems (facilitating immune signal 1) or director immunostimulatory molecules (facilitation immune signal 2) or a combination of both [
95].
The most common and long-time used immunoadjuvant alum has been extensively reviewed before [
100,
101], but mostly induces improved antibody responses. Other more recent clinical stage adjuvants include oil-based emulsions, such as MF-59, saponins, ISCOMs, oxoadenines, C-type lectin ligands. One prominent category includes Toll-like receptor agonists such as poly(I:C), the family of imidazoquinoline adjuvants (resiquimod, imiquimod), GLA, CpG motifs or flagellin. Another adjuvant group includes particle adjuvants, mimicking micro-organism structures, including nano- or microparticles or virus-mimicking polymers, such as polyphosphazene polyplexes PCPP and PCEP. Modularly composed AS0 adjuvants of Glaxo Smith Kline (GSK) and the CAF adjuvants of the Statens Serum Institute, have been extensively reviewed before [
103,
104]. All these systems will not be described in this review. Instead, a limited number of prototype examples of rationally designed vaccine adjuvants are described below.
3.2.1. Natural Lipid A as Source of Inspiration for New Adjuvants
The lipid A molecule is the immunostimulant component of Gram-negative bacterial lipopolysaccharide (LPS). It consists of a β-(1→6)-linked diglucosamine backbone with different patterns of acylation and phosphorylation [
105]. The lipid A structure activates the TLR4/MD-2 immune receptor complex that mediates gene expression and secretion of pro-inflammatory cytokines. Through activation and regulation of various dendritic cell functions TLR4 activation bridges innate and adaptive immunity by inducing signals that are vitally involved in the initiation of adaptive immune responses [
106]. TLR4 activation by lipid A is structure dependent. The bis-phosphorylated hexa-acylated lipid A species with a 4/2 distribution of the acyl chains (
E. coli type) is the most toxic and its recognition and binding by the TLR4/MD-2 binary complex can lead to sepsis. Changing the number and length of the acyl chains or the phosphate decorations decreases its recognition by the immune system receptor [
107,
108]. A vast diversity of lipid A molecules can be found as moieties of Gram-negative bacterial LPS. Acyl chains can be absent (de-acylation) or hydroxylated [
109] or more acyl chains can be added such as palmitate limiting immune system recognition [
110]. The removal of the anomeric phosphate group of the
E. coli type lipid A makes the molecule only moderately active. The phosphate groups can be absent or replaced by monosaccharides [
111,
112,
113,
114] or can also be decorated by phosphoethanolamine or cationic monosaccharides as 4-amino-4-deoxy-β-
l-arabinose to neutralize their negative charge altering immune recognition [
115,
116]. In addition, in nature, the glucosamine disaccharide can be replaced by the 2,3-diamino-2,3-dideoxy-
d-Glc disaccharide [
117,
118]. There is a vast number of lipid A molecules that are partial agonists and antagonists of TLR4. Particularly, gut microbiota have proven to be a tremendous library of lipid A molecules with different activities [
119]. Lipid A is also capable of activating C-type lectin receptors (CTLR) [
120], and caspases [
121,
122,
123,
124]. In addition, some atypical LPSs can activate TLR-2, although the chemical determinants responsible of this interaction are not yet clear [
113,
125].
For the structural reasons explained above, some natural LPS could be used as vaccine adjuvants. For example,
Brucella abortus LPS showed promising results in various formulations [
126,
127], as well as the naturally occurring monophosphorylated lipid A of
Bacteroides thetaiotaomicron and
Prevotella intermedia [
128]. There are also LPS-modified adjuvants like the
O-deacylated lipooligosaccharide from
E. coli J5 [
129] or the broadly used adjuvant Mono-Phosphoryl Lipid A (MPL) () [
130]. MPL is derived by the removal of the anomeric phosphate group of the hepta-acylated lipid A of
S. minnesota R595, which reduces significantly the TLR4-driven activity and toxicity. Decreased TLR4 activation induced by MPL derives from a less efficient dimerization of TLR4/MD-2/MPL complexes due to the absence of the phosphate group which the weakens proteins’ interaction at the dimerization interface [
131,
132,
133,
134]. MPL formulations are incorporated in approved vaccine preparations; moreover, MPL has also been used as a starting point of further modifications to develop new adjuvants [
135,
136].
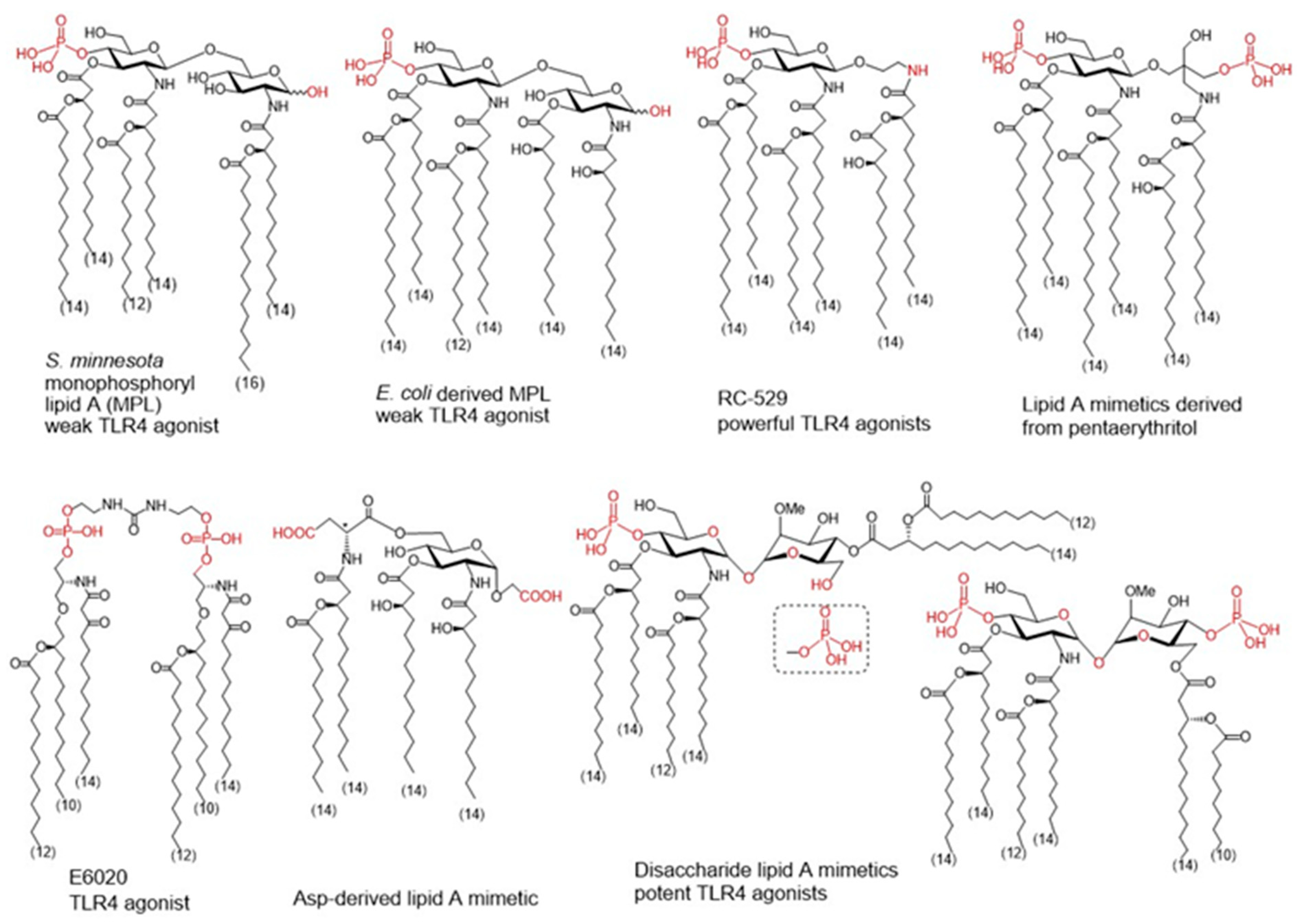
Figure 3. Monophpsphoryl lipids (MPLs)Ls and synthetic lipid A mimetics as promising vaccine adjuvant candidates.
To improve specific adjuvant properties and to modify or enhance immune stimulating activity, different synthetic lipid A analogues and lipid A mimetics have been prepared as vaccine adjuvant candidates. Chemical synthesis provides access to structurally defined molecules free of any biological contaminations and allows tremendous improvements in structure-activity relationships studies. Basically, all synthetic lipid A mimetics mirrored the basic architecture of their parent lipid A and were composed of a hydrophilic polar/charged head group and a hydrophobic lipid region. Simplification of the lipid A structure by replacing one or both glucosamine (GlcN) rings for linear or branched aglycons. Thus, GSK Biologicals developed aminoalkyl glucosaminide phosphates (AGPs) where the proximal GlcN ring of lipid A was omitted furnishing in this way a polar head group with rationalized structure [
137]. Variable β-hydroxyacyl and alkyl chains were chemically attached to the polar head group and selected AGPs reached pre-clinical/clinical development which highlighted RC-529 as a potent vaccine adjuvant () [
138]. Lipid A mimetics containing pentaerythritol in place of proximal GlcN residue were developed by Biomira Inc. (Edmonton, AB, Canada) as potent cytokine secreting agonists. A pentaerythritol-derived lipid A mimetic demonstrated adjuvant properties through enhancement of antigen-specific T cell activation in a synthetic liposomal vaccine system [
139]. Replacement of the distal GlcN moiety of lipid A for the acidic amino acid Asp and the glycosidic phosphate group for a carboxyl group (Asp-derived lipid A mimetic) ensured immunostimulating potential despite of only four lipid chains attached [
140]. Further structure simplification led to development of an acyclic lipid A mimetic E6020 (Eisai, Tokyo, Japan) consisting of a hexaacylated flexible linear backbone () [
141]. The antibody response to vaccines co-administered with this TLR4 agonist E6020 led to a mixed Th1/Th2 response [
142]. In addition, newly developed disaccharide lipid A mimetics profited from a conformational rigidity of their nonreducing disaccharide backbone and exhibited picomolar affinity for TLR4 [
143]. Thus, adjustable TLR4 activation and graded induction of cellular pro-inflammatory responses renders these glycolipids promising vaccine adjuvant candidates [
144].
3.2.2. Rational Design of Allostatine Adjuvant
Alloferons are a group of natural peptides isolated from insects that can stimulate human natural killer (NK)cell cytotoxicity towards cancer cells. These peptides are originally isolated from the hemolymph of maggots from the blowfly
Calliphora vicina. A striking feature of larval hemolymph is that the hemocytes possess a cytotoxic activity functionally analogous to human NK cells. When stimulated by bacteria, these larvae produce high levels of potent defensive molecules typical of the insect immune system which accumulate in the hemolymph [
145]. These include Alloferon1 and Alloferon2, two virtually indistinguishable peptides consisting of 13 and 12 amino acids respectively. Both Alloferons stimulate in vitro natural cytotoxicity of human blood mononuclear cells. Alloferon1 stimulates natural cytotoxicity in human in vitro models as well as antiviral and anticancer activities in mouse models in vivo. In a search for a molecule with higher antitumoral activity and cancer immunotherapy potential, the primary structure of Alloferon1 has been modified to obtain the peptide Allostatine, by changing two amino acids. The substitution of two amino acids in the Alloferon sequence was designed to simulate a pattern typical of the human immunoglobulin [
146] belonging to the immunoglobulin heavy chain CDR3 region which is very well conserved between mammalian genomes, notwithstanding that CDR3 region is the most variable section of immunoglobulins. The molecular mechanism of action of Allostatine is still not clear. In silico simulations suggested NKG2D as a possible target for this peptide, while in vitro experiments showed that low, ng/mL concentrations of Allostatine in culture medium cause rearrangement of NK and T cells receptors, stimulating NK cells cytotoxic activity against cancer cells increasing the number of IFN-gamma and IL2 producing cells [
147]. Allostatine manifested strong adjuvant properties in a mouse P388/DBA2 tumor transplantation model when combined with a vaccine consisting of X-ray inactivated tumor cells. While the vaccine alone demonstrated only a weak tumoristatic effect in about 25% of recipients, The vaccine in combination with Allostatine caused a tumoristatic effect in approximately 65% of recipients and prevented tumor occurrence in another 30% (resulting in a positive influence on 95% of recipients) [
146]. Hence, the designed peptide Allostatine therefore, possesses characteristics of an adjuvant boosting cancer therapeutic vaccines efficacy.
3.2.3. Nod2 Agonists as Rationally Designed Vaccine Adjuvants
Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is a cytoplasmic pattern recognition receptor involved in both innate as well as adaptive immune responses and therefore constitutes an excellent target for rationally designed vaccine adjuvant ligands [
148]. Muramyl dipeptide (MDP) is the smallest structural subunit of bacterial peptidoglycan capable of eliciting NOD2 activation, which leads to pro-inflammatory and antimicrobial responses characterized by the secretion of cytokines, induction of autophagy and production of antimicrobial peptides [
148]. Activation of NOD2 itself is sufficient to shape the adaptive immune response towards a Th2 response [
149]. Incidentally, NOD2 agonistic activities have been shown to strongly correlate with their adjuvant properties [
150]. NOD2 agonists also amplify the adjuvant potential of TLR ligands and alter the magnitude, persistence and the type of response towards the Th1 type [
151]. Interestingly, engagement of NOD2 proved to be essential for antigen-specific mucosal and systemic responses of mucosal vaccines [
152,
153]. This is noteworthy in light of the fact that the majority of pathogens gain entry through mucosal sites and given the shortage of mucosal adjuvants, the use of NOD2 agonists in cancer vaccines was also highlighted [
154].
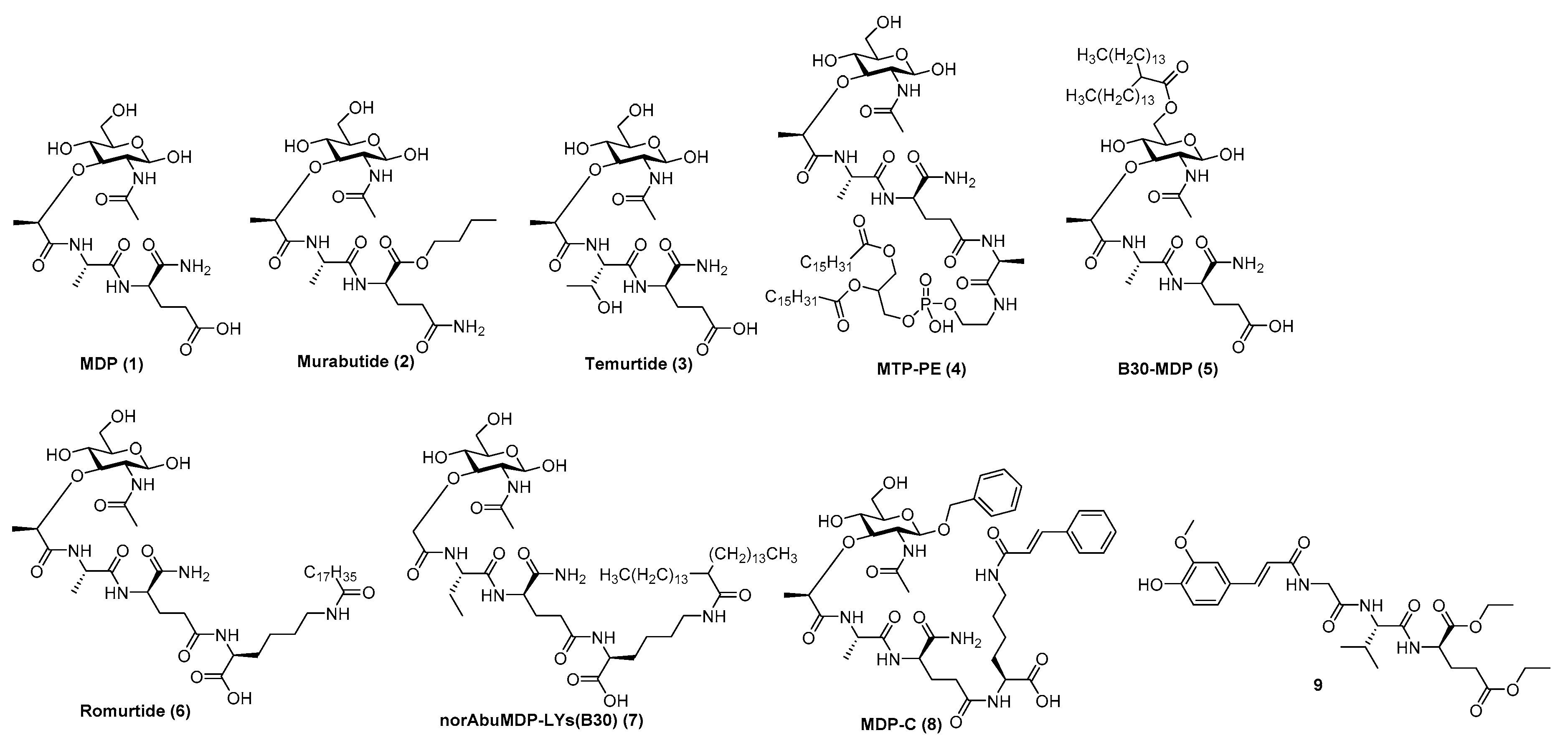
Although MDP ( (
1)) is predominantly responsible for the efficacy of Freund’s complete adjuvant, it suffers from pyrogenicity and rapid elimination as a single administrated molecule [
151]. To that end, many chemically modified derivatives of the parent MDP molecule () have been synthesized with the aim of reducing its toxicity and improve its pharmacokinetic properties. Of the hydrophilic derivatives known to mainly induce a Th2-type response, murabutide ( (
2) and temurtide ( (
3) emerged as the most interesting candidates for further development as vaccine adjuvants, while muramyl tripeptide phosphatidylethanolamine (mifamurtide; MTP-PE) ( (
4), B30-MDP ( (
5) and romurtide (MDP-Lys (L18) ((
6) were the most prominent lipophilic derivatives, which tend to augment the Th1-type immune reaction [
154].
Figure 4. MDP (1), its most prominent pharmacologically active derivatives (2–8) and a desmuramylpeptide derivative with NOD2 activity (9).
Substitution of the
N-acetyl group by an
N-glycolyl group produces
N-glycolyl-MDP, a distinctive feature of the BCG vaccine, which exhibits superior adjuvant activity. The
l-Ala position is also available to other amino acids, since
l-Ser,
l-Val and
l-Thr peptide analogs retained the adjuvant activity of the parent molecule [
150]. The
d-iGln moiety, on the other hand, is less amenable to substitution; it can only be replaced by either
d-Gln or
d-Glu (including their esterified forms) as exemplified by murabutide or muradimetide [
148]. Murabutide is apyrogenic and well tolerated by humans [
155], therefore its adjuvant effect was assessed following administration with the fluid phase of tetanus toxoid vaccine, in which case significantly higher IgG levels to toxoid were found in the group receiving vaccine with murabutide compared to the group given the vaccine alone [
156]. Its adjuvant capacities were further underlined using a combination of murabutide and a synthetic hepatitis B antigen with increased levels of antigen-specific antibodies [
157]. Recently, Jackson et al. reported on the ability of murabutide to induce a robust and durable IgG and IgA antibody response to Norwalk virus following intranasal vaccination which proved its ability to act as a potent mucosal adjuvant [
153]. Temurtide is a threonine-based MDP derivative, used as an active principle of the SAF-1 formulation, which has been tested as adjuvant in preclinical trials in guinea pigs and mice. It successfully increased the formation of IgG2a antibodies against HBsAg [
158].
The introduction of lipophilic groups into the structures of NOD2 agonists has been shown to strongly enhance the cellular immune response and overall increased the immunostimulatory adjuvant activity of the compounds. MTP-PE ((
4) was evaluated in Phase I clinical trials as a vaccine adjuvant in human immunodeficiency virus type I vaccines, but failed to improve their immunogenicity, while causing increased reactogenicity [
159]. Decoration of the muramyl residue 6-OH group with a lipophilic linear/branched fatty acid structural feature resulted in derivatives with noteworthy adjuvant properties, as exemplified by B30-MDP [
148]. Further structural optimization, which entailed the extension of the peptide stem with
N-stearoyl-
l-Lys, led to the discovery of MDP-Lys(L18) (also known as romurtide or muroctasine). A combination of MDP-Lys(L18) and B30-MDP has shown promising results in mice, which produced higher antibody titres against rHBsAg after intraperitoneal injection [
160] and increased the humoral and cellular response against an inactivated hantavirus vaccine [
161]. A close structural analog norAbuMDP-Lys(B30) ( (
7), which proved effective as an adjuvant for
Borrelia burgdorferi antigen rOspA, also features a B30-acylated Lys-extension [
162]. Similarly, MDP-C ( (
8) carries a
N-cinnamoyl-
l-Lys moiety and showed promising results in a mouse model by increasing the levels of anti-HBs antibodies [
163]. Desmuramylpeptides are MDP derivatives in which the sugar
N-acetylmuramyl moiety (Mur
NAc) is replaced by a hydrophobic group. The
Trans-feruloyl moiety has recently been identified as an excellent Mur
NAc mimetic, resulting in a low nanomolar NOD2 agonist ( (
9), which induced ovalbumin-specific IgG titers in a mouse model of adjuvancy [
164].
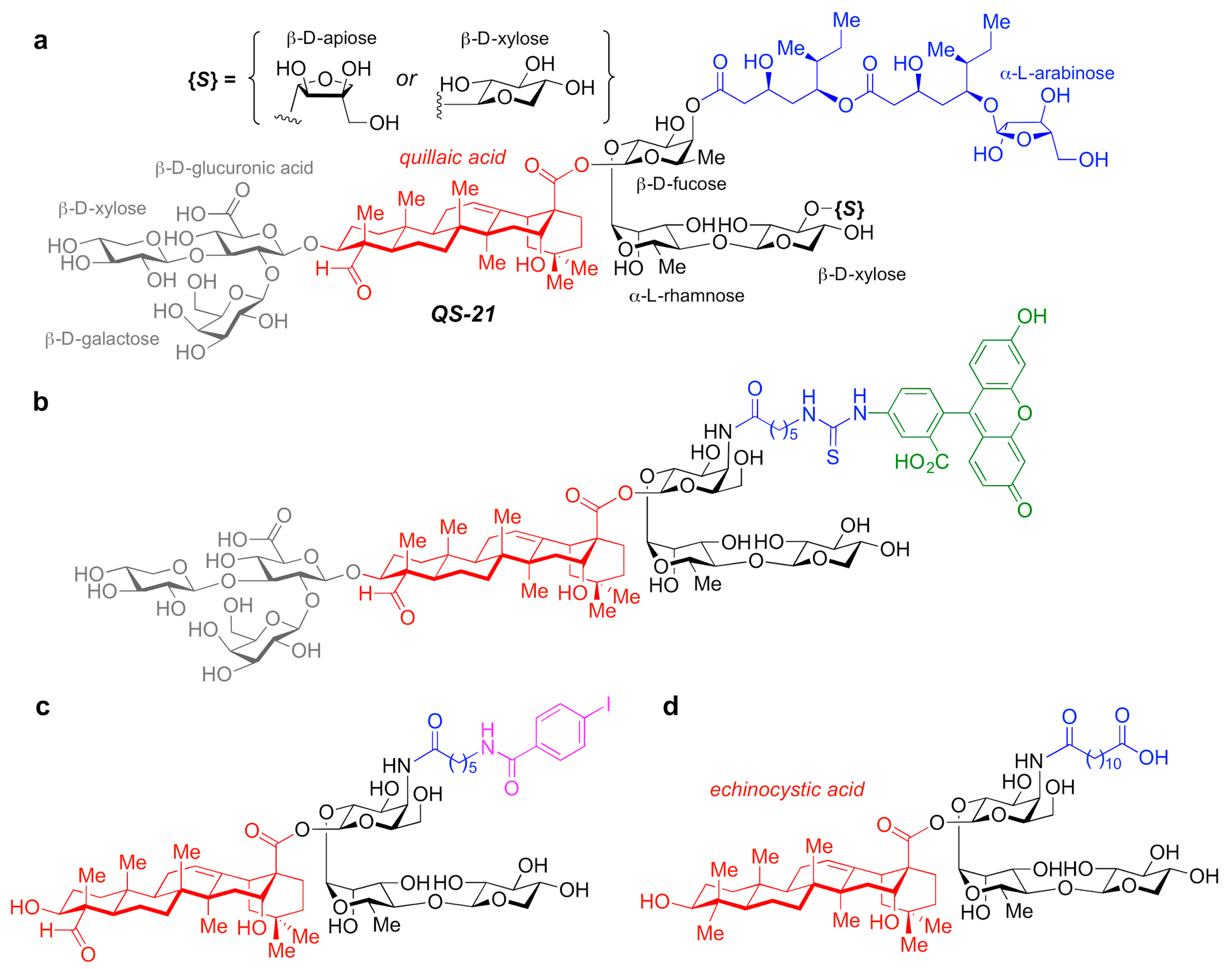
3.2.4. QS-21-Based Synthetic Saponin Adjuvants
A number of adjuvant analogues have been developed based on natural product adjuvants leveraging detailed structure–activity relationship studies, such as synthetic saponin variants derived from QS-21 (a) and the
Quillaja saponin (QS) family [
165].
Figure 5. (a) Chemical structure of QS-21 saponin natural product adjuvant. (b–d) Synthetic saponin variants based on QS-21.
In particular, QS-21 is a saponin natural product with a long history and great potential as an adjuvant. It elicits both antibody and cellular immune responses, including cytotoxic T lymphocytes, and has been recently approved in combination with MPLA as part of the AS01 adjuvant system in vaccines against malaria and shingles [
166]. However, despite its promise, QS-21 suffers from several drawbacks, including scarcity, heterogeneity, chemical instability and dose-limiting toxicity, which have hampered its more widespread use in human vaccines. As such, the discovery of new, improved QS-21 variants has been at the forefront. In this context, Fernández-Tejada et al. identified key structural features of QS-21 that are important for adjuvant activity [
167,
168] and have developed a variety of simplified, synthetically accessible saponin derivatives that induce antibody responses comparable to QS-21 with drastically reduced toxicity (b–d) [
169,
170]. Moreover, these structurally simpler saponin scaffolds were leveraged for the development of saponin variants bearing fluorescent and radioiodinated tags (b,c). These saponin probes were exploited in imaging and biodistribution studies that revealed internalization of active variants into dendritic cells and accumulation in the lymph nodes, which suggests a role for adjuvant-active QS variants in the trafficking of antigens by APCs to the draining lymph nodes [
169].
Additional studies on the detailed immunological profile of these saponins are warranted and in progress, with the aim to elucidate the precise functional and molecular roles of these adjuvants, also in the context of adjuvant systems and anti-cancer vaccines.