Xanthophylls (fucoxanthin, astaxanthin, lutein, zeaxanthin, and β-cryptoxanthin) are a type of carotenoids with anti-tumor and anti-inflammatory activities, due to their chemical structure rich in double bonds that provides them with antioxidant properties.
- carotenoids
- xanthophylls
- natural compounds
- algae
- bioactive
- health
1. Introduction
In recent years, consumer demand for naturally sourced products to promote health and reduce disease has grown steadily [1]. This demand has entailed an increased interest in new natural sources of food, pharmaceutical, and cosmetic products [2,3]. In this context, the marine environment has been considered a potential reservoir of natural compounds [4]. Among the organisms present in this environment, it is worth highlighting algae. Algae constitute a polyphyletic group of photosynthetic primary producers organisms, which represent an interesting source of chemical components with high-value biological activities. [5]. Although the total number of algal species is unknown, it is thought to vary between one and ten million [6].
The high value of algal extracts is due to their large number of molecules such as carbohydrates, proteins, peptides, lipids (including oils and polyunsaturated fatty acids, PUFAs), minerals, iodine, phenols (polyphenols, tocopherols), alkaloids, terpenes, and pigments (as chlorophylls, carotenoids, and phycobilins) [7,8]. Within these compounds, one of the groups with greater interest are pigments due to the concentrations in which they are present, these being higher than that of other compounds such as phenolic compounds. In fact, algae are considered pigment-producing organisms. They have a great variety of pigments, which can be classified into three large groups: chlorophylls, carotenoids, and phycobilins. Therefore, different carotenoids (CA) profiles can be used as a medium for algal classification [9]. In this way, a first classification of the algae allows us to make a division according to the size of the algae (microalgae or macroalgae) and the following divisions according to their tones, among other characteristics. As a result, the first group comprises greenish algae (Cyanophyceae), green algae (Chlorophyceae), diatoms (Bacillariophyceae), and golden algae (Chrysophyceae), among others. Meanwhile, the macroalgae family includes red (Rhodophyta), brown (Ochrophyta), and green algae (Chlorophyta) [10,11,12]. This diversity of species and, therefore, of its chemical compositions is interesting, since once compounds are properly isolated or extracted from algae, they may show a diverse range of biological activities, such as antioxidant, antimicrobial, anticancer, anti-allergic, antiviral, and anticoagulant activities, among others [7,8]. This diversity of biological activities implies that there is also a significant variety of potential applications in human health, agriculture, and in food and cosmetic industries [4], in which its application depends on its chemical composition.
On an industrial scale, the most interesting species are those that produce high percentages of CA. CA are usually located in chloroplasts or stored in vesicles and a cytoplasmic matrix of plants, algae, photosynthetic bacteria, and some fungi [9]. All CA are tetraterpenes, which are compounds that have a skeleton composed of 40 carbon atoms conjugated in polyene chains [9]. They are classified into two main groups: (i) compounds that have a hydrocarbon long chain known as carotenes and (ii) compounds that have an oxygen atom in its structure, known as xanthophylls. The first group includes α-carotene, β-carotene, lycopene, and phytoene, among others. The most representative molecules of the second group are fucoxanthin, astaxanthin, lutein, zeaxanthin, and β-cryptoxanthin. This difference in its structure makes xanthophylls more polar than carotenes due to the presence of oxygen in the form of methoxy, hydroxy, keto, carboxy, and epoxy positions. However, except for lutein, they are still non-polar compounds [13]. Its structure with alternating double bonds is responsible for many of its biological functions, being the main function in photosynthetic organisms to act as accessory pigments for the capture of light in photosynthesis, and to protect photosynthetic machinery against self-oxidation [14]. However, despite the wide diversity of molecules in the carotenoid family, with more than 700 compounds currently known, only about 30 CA have a significant role in photosynthesis [13]. In recent years, numerous studies have highlighted CA multiple effects on human health due to their antioxidant properties, preventing the damage caused by oxidative stress and therefore declining the risk of chronic diseases [14,15]. However, the biological properties of CA are not limited to their antioxidant properties. The scientific literature has shown CA actions as anti-tumor [16,17,18], anti-inflammatory [19,20], neuroprotective, antimicrobial, antidiabetic, and antiobesity [21,22]. Therefore, algae have several CA with market interest (β-carotene, fucoxanthin, astaxanthin, lutein, zeaxanthin, and violaxanthin), representing a natural and sustainable source of these compounds [9].
Among the xanthophylls of interest is fucoxanthin, which is one of the most abundant marine CA, accounting for approximately 10% of the total production of natural CA [23]. It is found in abundant concentrations in the chloroplasts of several brown seaweeds, such as Laminaria japonica, Undaria pinnatifida, Sargassum fusiformis, in several species belonging to the genera Sargassum (Sargassum horneri) and Fucus (Fucus serratus, Fucus vesiculosus) and in diatoms (Bacillariophyta) [9,24,25,26]. Another xanthophyll of interest is astaxanthin (AS), which is a red pigment. AS is considered a potent antioxidant as it has about ten times more antioxidant activity than other CA [27]. The main natural sources of this pigment are the microalgae Haematococcus pluvialis, Chlorella zofingiensis, and Chlorococcum sp. [28]. H. pluvialis is a single-celled green freshwater alga. It is the richest source of natural AS and is already produced on an industrial scale [26]. Procedures have been technologically advanced to grow Haematococcus containing 1.5–3.0% AS dry weight [27,29]. The richest source of β-carotene is the halotolerant green microalgae Dunaliella salina, accumulating up to 10% of it based on the dry weight of the microalgae [30,31]. When H. pluvialis and D. salina are cultivated in extreme conditions (such as high salinity, high luminosity, or lack of nutrients), AS and β-carotene, respectively, can reach more than 90% of the total carotenoids [7]. Lutein and zeaxanthin are pigments found in algal species such as Scenedesmus spp., Chlorella spp., Rhodophyta spp., or Spirulina spp. respectively [32]. Esteban et al., 2009 [33], reported that red algae (Rhodophyta) show a common carotenoid pattern of β-carotene and one to three xanthophylls: lutein, zeaxanthin, or anteraxanthin. Corallina elongata and Jania rubenseran were the only algae that contained anteraxanthin as the main xanthophyll. Spirulina platensis (strain pacifica) microalgae is a source of β-cryptoxantine, β-carotene, and zeaxanthin. β-cryptoxantine is a pigment that can also be found in plants [34]. The siphonaxanthin content in green algae such as Umbraulva japonica, Caulerpa lentillifera, and Codium fragile constitutes about 0.03%–0.1% of the dry weight [35]. The cyanobacteria Synechococcus sp. strain PCC7002 produces a monocyclic myxoxanthophyll, which is identified as Myxol-2 Fucoside (Myxoxanthophyll), in addition to producing other CA such as β-carotene, zeaxanthin, and sinecoxanthin [36]. The CA composition in cyanobacteria is very different from that of other algae, including for example β-carotene, zeaxanthin, myxol pentosides, and echineone [32].
Animals should get all these CA through the diet, as they are unable to synthesize them. CA are commonly incorporated as dietary supplements, feed additives, and food colorants in several sorts of food, such as dairy products and beverages, and also in the pharmaceutical and cosmetic industries [37]. As shown in Figure 1, CA have a high repertoire of commercial applications due to their multiple biological properties. Among the most notable applications are cosmetic, nutraceuticals, pharmaceutical purposes, and other human applications.
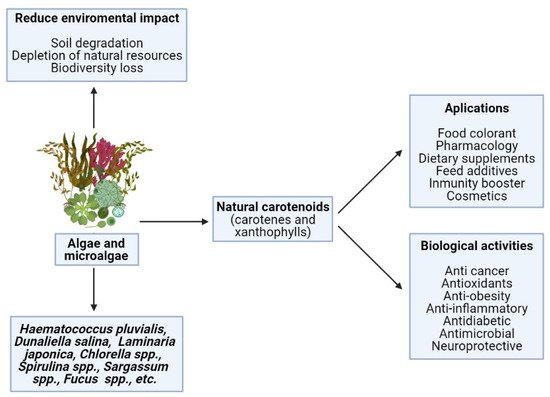
Figure 1. Positive effects on human health and industrial applications of carotenoids from natural sources.
Attributable to the various positive activities on human health and the multiple industrial applications of CA, global demand continues to increase. It is estimated that in 2026, the CA market will grow to USD 2.0 billion, registering an annual growth rate for CA of 4.2% [38]. The most relevant and important pigments on the market today are β-carotene and AS, followed by lutein, lycopene, and canthaxanthin [13,31]. So far, most commercial CA are artificially produced. However, the strong global interest in food of natural origin that is safe, healthy, and environmentally friendly has increased the demand for natural sources of CA [22]. Algae and algal extracts are a sustainable option for CA and have numerous benefits in comparation to alternative natural sources. For instance, its cultivation and production is cheap, easy, and ecological, its removal has higher yields and is simple, and raw materials are not scarce, nor are there seasonal limitations [32,39,40]. In order to obtain high concentrations of a certain compound, culture conditions and environmental stress can be modified to manipulate the biochemical composition of microalgae [39]. However, under optimal growth conditions, the concentration of CA pigments is often too low to produce microalgal-based pigments, making it economically unviable [13,40]. To improve its economic viability, it is vital to explore and understand how environmental factors and the integration of nutrients into the environment affect the production of compounds. Understanding how the metabolic pathways of species vary according to the culture conditions, the co-production and accumulation of multiple compounds in microalgae will be improved [41].
2. Mechanism of Action of Xanthophylls
2.1. Metabolism
The mechanism of action of xanthophylls is the specific binding through which the molecule produces its pharmacological effect. This effect will depend on the absorption, distribution, and metabolism of the compound, which are critical parameters of the pharmacokinetics of the xanthophylls. This can be seen in various studies that show the low presence of this type of compound in human tissues, which directly depends on their metabolism and intestinal absorption, and therefore, its bioavailability [143]. The metabolism of xanthophylls is poorly studied, especially for those that do not have provitamin A activity. Hence, more studies are needed to understand its metabolism and, therefore, be able to develop different applications according to the mechanism by which its biological activities occur.
In turn, this would allow the development of safe and effective applications in humans as well as increase its bioavailability [144]. For example, studies on FU metabolism revealed that this compounds itself is not present in plasma but rather its metabolites due to oxidative reactions that take place on FU in mammals. This reaction transforms both compounds into ketocarotenoids [145]. In addition, when FU is administered orally, it undergoes a process of hydrolysis at the intestinal level, giving rise to fucoxanthinol, while liver metabolization results in other active metabolites such as amarouciaxanthin A [146,147]. In fact, it was reported that dietary FU accumulated in the heart and liver as fucoxanthinol and in adipose tissue as amarouciaxanthin A, the latter being non-detectable by HPLC in human serum [148]. Therefore, the oral administration of this compound may only provide some bioactive metabolites, as it is completely metabolized. To release products that maintain its biological activities, it is necessary to develop alternatives that prolong its biological half-life [146], such as emulsions or encapsulations (Table 2).
Table 2. Delivery systems used to increase marine carotenoids’ bioavailability.
| Mol. | Delivery System | Assay | Benefits | Results | Use | Ref. |
|---|---|---|---|---|---|---|
| FU | Palm stearin solid lipid core | In vitro | Increase stability during storage | Release of FU of 22.92% during 2 h in SGF and 56.55% during 6 h SIF | Oral supplements | [149] |
| Nanoparticles of zein | ABTS DPPH | Increase antioxidant activity | More antioxidant than free FU | Foods and beverages | [150] | |
| Nanoemulsion | In vitro | Increase stability during storage; antiobesity | 95% of FU remains in the emulsion after 4 weeks | Food, beverages, nutraceutics | [151] | |
| Nanoemulsion (LCT) | In vitro digestion and bioability assays in rats | Increase stability | Increase FU level in serum blood (LCT > MCT) | Functional foods and nutraceutics | [152] | |
| Chitosan–glycolipid nanogels | In vitro | Significant increase in bioavailability | Lpx levels (nmol MDA/mL) higher in control (30.9) than in emulsions (17.0–12.15) | Foods and nutraceutics | [153] | |
| AS | Fish oil | In vitro | Useful for supplementation | Better antioxidant effect | Oral supplements | [154] |
| Encapsulation | TBARS Peroxide enzymes | Increase stability | Better antioxidant effect | Foods | [155] | |
| Pectin–chitosan multilayer | Stability Assays | Increase stability | Better stability than monolayer | Nutraceuticals, functional, medical foods | [156] | |
| l-lacic acid | Release and stability test | Increase stability | Enhance stability | Functional foods and nutraceutics | [157] | |
| Ascobyl palmitate emulsion | Stability assay | Sublingual delivery | Enhance sports performance, skin protection, cardioprotective | Dietetic supplementation in sports | [158] | |
| LU | β-CD | In vitro | Increase stability | More stable against oxidating agents | Foods | [159] |
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [159] | |
| ZEA | Sea Buckthorn oil and water emulsion | Stability and digestive assays | Increase bioaccesibility | Increase 64.55% | Functional foods and nutraceutics | [160] |
| High-pressure treatment | Stability and digestive assays | Improve Nannochloropsis sp. ZEA disponibility | Foods | [161] | ||
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [159] |
SGF: Simulated gastric fluid; SIF: Simulated intestinal fluid; LCT: Long-chain triglycerides; MCT: Medium-chain triglycerides.
A study carried out on rats reported that the pharmacokinetic parameters of AS only depend on the dose when it is administered intravenously due to the metabolism that takes place in the liver as a result of saturation of hepatic metabolism of AS [162]. As for AS metabolites described in humans, these are fundamentally 3-hydroxy-4-oxo-β-ionone and 3-hydroxy-4-oxo-7,8-dihydro-β-ionone [163]. The metabolization of AS after oral intake leads to 3-hydroxy-4-oxo-7,8-dihydro-β-ionol and 3-hydroxy-4-oxo-7,8-dihydro-β-ionone, being both compounds detected in plasma [164]. Several researchers hypothesize that the rate at which these reactions take place is determined by the structure of the ring, as well as by the length of the fatty acyl residue formed. Moreover, several enzymes, such as for example diacylglycerol acyltransferase 1, can catalyze the synthesis of AS esters in some strain. This is the case of the microalga Haematococcus pluvialis [165].
As for LU and its structural isomer, ZEA, studies carried out in humans have shown that both undergo an in vivo oxidation process that gives rise to several metabolites [166]. LU gives rise to a series of compounds (3′-epilutein, 3′-oxolutein) due to the presence of the enzyme that also mediated the conversion of fucoxanthinol to amarouciaxanthin A [167]. Other compounds such as 3-hydroxy-3′,4′-didehydro-β,γ-carotene and 3-hydroxy-2′,3′-didehydro-β,ε-carotene appear as result of acid hydrolysis in the stomach [168]. However, this compound is capable of remaining intact in its intact form in human ocular tissue due to the inability of the enzyme β-carotene-9′,10′-oxygenase to act on said organ. In this way, there is an extraordinary accumulation of these compounds in the ocular tissue, serving as a mechanism for the prevention of ocular diseases [169]. ZEA, being an isomer of LU, will undergo similar processes to LU. However, it is a much less studied molecule. In this way, ZEA will also be accumulated in the ocular tissue due to the inactivity of the enzymes responsible for the metabolism of ZEA in the organs of sight [170]. Therefore, to determine the bioavailability of LU it is necessary to quantify said metabolites, which also may have different bioactivities, with complementary studies.
2.2. Bioavailability and Bioaccessibility
Xanthophylls have been subjected to numerous studies due to its antioxidant activity and protective effect against several diseases [171]. In recent years, different studies have been carried out comparing the properties of synthetic CA with those of natural origin [172], noting that some of them can only be obtained from natural sources, where there is much more diversity. In addition, these CA obtained from algae can be co-extracted with other bioactive components such as polysaccharides or fatty acids. Therefore, the idea of incorporating CA in foods, nutraceuticals, or cosmetic products is of increasing interest due to their effective bioactive properties [173]. However, to develop and evaluate the viability of any food or cosmetic products that maintain these activities, it is necessary to know its bioactivity, bioavailability, and bioaccesibility [174]. These three parameters are influenced by several factors such as the food matrix; the type of cooking; the time of cooking; the CA involved; the presence of fats, fibers, proteins, and other nutrients in the diet; and the health or nutritional status in humans [175,176,177,178,179].
In humans, once CA are ingested, they are released from the food matrix through the action of gastric enzymes and must be emulsified with lipids in order to improve their absorption [180]. Moreover, its absorption mechanism will be determined by the concentration in which the compound is present. At low concentrations, absorption is mainly due to the action of type 1 class B scavenger receptor, which also captures high-density lipoproteins, platelet glycoprotein 4, and NPC1-like intracellular cholesterol transporter 1 [181]. At high concentrations, the main mechanism is passive diffusion through mucosa [182]. Enzymes released in the duodenum will also play an important role in the absorption, since in this part of the small intestine, pancreatic lipase is released. This enzyme assists the formation of mixed micelles of emulsified droplets with CA. This process depends on the concentration of bile acids among others [183]. Once the micelles are formed, they pass into the blood. Then, micelles are taken up by enterocytes, in which metabolization takes places due to the presence of the enzyme β-carotene oxygenase. The non-metabolized CA, such as LU and ZEA, are incorporated into chylomicrons or low-density lipoproteins (LDL) and are transported to the liver where they can be eliminated by the bile or metabolized and secreted in very low-density lipoprotein (VLDL) or high-density lipoproteins (HDL) to the peripheral tissues, as it can be seen in Figure 3 [180,184].
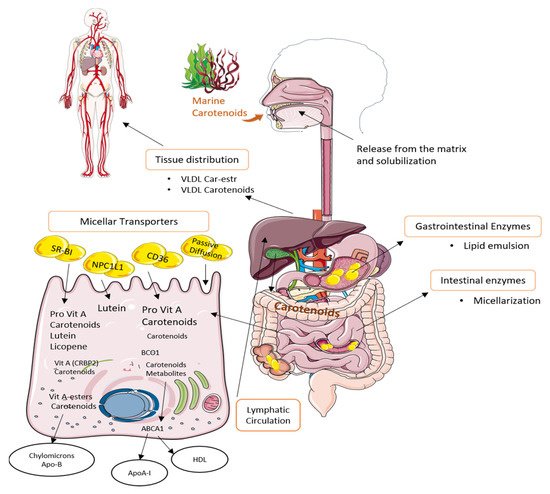
Figure 3. Uptake, transport, and secretion pathways of marine carotenoids in the human body.
All these absorption processes involve passing through membranes, which will be determined by the polarity of the membrane and the compounds. CA are frequently esterified with fatty acids, which decreases the polarity, so except for lutein, they are considered non-polar molecules. Among CA, xanthophylls have a bit higher polarity than carotenes. This is due to the small number of oxygen atoms in their structure (Figure 2). In addition, the polar groups of the molecules are at opposite ends of the molecule, so their forces cancel out. Therefore, the presence of hydroxyl groups makes them a bit more polar than carotenes, which do not contain oxygen but are still considered non-polar molecules [185]. CA polarity and flexibility seem to be correlate with bioaccessibility and uptake efficiency. This may be due to the fact that this type of CA presents a better affinity for lipid transporters and/or for plasma membranes, which would increase absorption [186]. Therefore, these compounds may be the CA with highest bioavailability. Different mechanisms have also been developed to increase the bioavailability of these compounds, of which the most common are the elaboration of emulsions or encapsulations.
3.2.1. Fucoxanthin
Different in vitro, in vivo, and clinical studies show that FU digestion and absorption gives rise to metabolites such as fucoxanthinol. In a study carried out with mice, FU was transformed into fucoxanthinol in the gastrointestinal mucosa by deacetylation due to the action of lipase and cholesterol esterase enzymes. Then, the fucoxanthinol that reached the liver was transformed to amarouciaxanthin by deoxidation. As a result, fucoxanthinol could be detected in the heart, spleen, liver, and lung, and amarouciaxanthin could be found in adipose tissue [145,148]. During all this process, pH is a limiting factor since, as it was observed in an in vitro simulated digestion study, enzymes can be inactivated due to low pH and, consequently, FU would remain intact [187]. A study of the colonic fermentation of FU reported that 50% of FU can be metabolized by action of the human microbiota, ensuring that the compound is bioaccessible [187]. However, the absorption of FU is lower than the rest of the CA despite achieving better accumulation [188]. This may be due to digestion of the compound. In fact, FU supplementation in adults correlated with fucoxanthinol increase in serum [189]. A human trial carried out with FU extracted from Undaria pinnatifida concluded that after the supplementation of an extract with 6.1 mg of FU, FU could not be detected in blood, and the metabolite fucoxanthinol was at very low concentration, which confirms the limited intestinal absorption of FU [190]. In order to improve its absorption, different mechanisms have been developed, of which the most common encapsulation is in micelles or liposomes [149]. The best results are obtained when long or medium-chain triglycerides are used to carry out the encapsulation [152]. Encapsulation can also be done with chitosan-glycolipid nanogels, which increase FU bioavailability by 68% according to in vitro studies [153]. Other options include encapsulation with proteins such as zein and caseinate, which provide better stability to FU and enhance its antioxidant and anti-tumor activity compared to free FU [150]. Yet, human studies are scarce and contradictory, since numerous factors that influence bioavailability are reported, such as the dietary fiber of the food matrix; the interaction with other nutrients such as lipids and proteins; the solubility of the molecule; or the affinity with intestinal transporters.
3.2.2. Astaxanthin
3.2.3. β-Cryptoxanthin
3.2.4. Zeaxanthin
3.3. Experimental Studies
The effects of CA on health have been long studied. As mentioned, some CA such as β-cryptoxanthin or β-carotene are precursors of retinol (vitamin A), while others such as fucoxanthin, lutein, or lycopene are not. As such, their intake relates to their role in retinol production, and to their antioxidant, anti-inflammatory, and anti-tumor activities [207]. In this regard, several in vitro as well as in vivo and observational or epidemiologic studies have been carried out in the last decades. Furthermore, the antioxidant role of CA has been long-known and evidenced for its use as antioxidant additive as well as antioxidant test assay [208]. The great majority of studies have assessed the intake of CA to test their effects, as it is the major ingress pathway of these molecules. As with other antioxidants of natural origin with observed health-promoting properties, it has been suggested that the potential chemopreventive effects of these molecules are derived from the synergy of their antioxidant and anti-inflammatory properties, besides their direct inhibition of certain factors involved in cell cycle and apoptosis [209]. This is due to the intimate relationship of oxidative stress as both a cause and result of inflammation and their relationship toward developing cancer [210,211]. Hence, the properties and effectiveness of CA have been tested and evaluated through various ways, both with molecular methods and relating their intake or serum levels with disease or mortality incidence. A summary of relevant findings will be addressed. Experimental designs and outcomes are shown in Table 3.
Table 3. Summary of studies and meta-analysis on the health-related properties and effects of carotenoids and observed results.
| Study | Model | Dose | Experimental Design | Observations | Ref. |
|---|---|---|---|---|---|
| Fucoxanthin | |||||
| Anti-inflammatory | In vitro. RAW 264.7 macrophages with LPS-induced inflammation | 15–60 μM | Expression of inflammatory mediators | D-d reduction of expression of IL6-IL-1, NO, and TNF-α | [212] |
| In vitro (Apo-9′). RAW 264.7 macrophages and zebrafish model | 25–100 μg/mL | Reduction of LPS-induced inflammation | D-d reduction of NO, ROS, TNF-α, and COX production | [213] | |
| In vitro and in vivo. RAW 264.7 and aqueous humor of rats | 10 mg/kg | Reduction of LPS-induced inflammation | D-d reduction of PGE2, NO, TNF-α by inhibiting iNOS and COX-2 | [214] | |
| Anti-cancer | Ex vivo. B16F10 cell culture implanted in mice | 200 μM | Growth inhibition of melanoma | D-d growth inhibition by inducing G0/G1 cell cycle arrest and apoptosis; inhibition production of retinoblastoma protein | [215] |
| In vitro. Human leukemic HL-60 cells | 15.2 μM | Inhibited the proliferation | DNA fragmentation | [216] | |
| Astaxanthin | |||||
| Anti-inflammatory | In vitro. RAW 264.7, splenocytes, and bone-narrow macrophages | 25 μM | Expression of inflammatory mediators in LPS-induced inflammation | D-d significant reduction of IL-6, IL-1β, and ROS production | [217] |
| In vivo. Mice with induced acute lung injury | 60 mg/kg/day for 14 days | Analysis of inflammation markers, tissue damage | Significant reduction of mortality, histological damage, inflammatory infiltration, and iNOS and NF-κβ levels | [218] | |
| Anti-cancer | In vitro. Human colon cancer lines HCT-116, SW480, WiDr, HT-29 and LS-174 | 5–25 µg/mL | Growth inhibition of with H. pluvialis astaxanthin-rich extract | D-d cell cycle arrest and apoptosis induction by lowering expression of Bcl-2, AKT and induced expression of apoptotic MAPK | [219] |
| In vivo. Chemically induced colitis and colon carcinogenesis mice | 200 ppm | Analysis of inflammatory biomarkers | D-d inhibition of NF-κβ, TNF-α, IL-1β, IL-6, and COX-2 expression; lower iNOS expression at high dosage | [220] | |
| Lutein | |||||
| Anti-inflammatory | Observational study. Early atherosclerosis patients (n = 65) | 20 mg/day for 3 months | Differences in serum cytokines, and metabolic biomarkers | Significant reduction in serum IL-6 MCP-1 and LDL-cholesterol after 3 months of supplementation | [221] |
| Observational study. Preterm infants (n = 203) | 30 mL/ kg/ day until 40 weeks post-menstrual age | Differences in inflammation biomarkers | Enhanced retinal development and reduced C-reactive protein levels | [222] | |
| Anti-cancer | In vivo. Rats | 3–30 g/L | Inhibition of N-methylnitrosourea-induced colon crypt foci formation | Significantly lowered formation of aberrant crypt foci | [223] |
| β-cryptoxanthin | |||||
| Anti-cancer | Prospective cohort study. Smokers and non-smokers from NHANES III (n = 10,382) | Dietary contribution | 20-year cohort | Higher serum levels of β-CRY were associated with lower death risk, but not for non-smokers | [224,225] |
| Ex vivo. Human gastric cell lines AGS and SGC-7901 implanted in mice | 0–40μM | Growth and proliferation inhibition | D-d growth and proliferation inhibitory activity by reducing cyclins, endothelial growth factor, PKA and increasing cleaved caspases expression | [226] | |
| In vivo. Mice | 10 mg/kg diet | Induced emphysema and lung tumorigenesis | D-d tumor mass reduction, decreased levels of IL-6 and AKT and restoration of silenced tumor-suppressor genes | [227] | |
| In vivo. Cigarette smoke-exposed ferrets | 7.5–37.5 μg/kg/day | Inflammation biomarkers and tissue damage analysis | D-d inhibition of NF-κβ, TNF-α, AP-1 expression as well as lung tissue squamous metaplasia and inflammation | [228] | |
| Siphonaxanthin | |||||
| Anti-cancer | In vitro. Human leukemia (HL-60) cells | 5–20 μM | Analysis on cell viability and apoptosis | D-d reduction of cell viability and induction of apoptosis by increasing levels of DR5, lower expression of Bcl-2 and increase in caspase-3 | [129] |
D-d: Dose-dependent; LPS: Lipoplysaccharide, ROS: Reactive oxygen species, IL: Interleukin, NRF2: Nuclear factor E2-related factor 2, PKA: Protein kinase A, AKT: Protein kinase B, ERK: Extracellular signal-regulated kinase, PAI-1: Plasminogen activator inhibitor-1, MMP: Metalloproteinases, Bcl-2: B-cell lymphoma 2, PG: Prostaglandin, RR: Relative risk, CI: Confidence interval.
3.3.1. Observation In Vitro
In vitro experiments testing properties of CA are of great value to analyze the role of specific molecules and discern potential participating molecules. Their apparent results have been reinforced in multiple animals and human studies, while in some cases, results have been mixed. In fact, most experiments with CA have been made in vitro. The in vitro studies analyzed in this article can be divided into two large groups. The first corresponds to those methods that quantify the antioxidant properties of xanthophylls. The second group includes those anti-inflammatory or anti-cancer tests in cell cultures. Inflammatory models usually comprise the use of human or murine macrophage cell cultures and measure differences in the expression or translation of pro-inflammatory mediators such as cytokines (tumor necrosis factor alpha (TNF-α), interleukins (IL)-1β and IL-6), nuclear factor (NF)-κβ (which mediates the expression of these cytokines), and the production of nitric oxide (NO) or enzymes related to the inflammatory process (cyclooxygenase (COX)-2, nitric oxide synthase (iNOS)) [209]. A study on RAW 264.7 murine macrophages, splenocytes, and bone marrow-derived mice macrophages obtained from mice fed with AS reported a significant reduction of IL-1β and IL-6 and generated ROS. Moreover, the authors described that AS inhibit nuclear translocation of NF-κβ and increase the expression of nuclear factor E2-related factor (NRF)-2, which subsequently involves a lower production of reactive oxygen species (ROS) and inflammatory response [217]. Experiments involving FU or some of its metabolites such as fucoxanthinol or apo-9′-fucoxanthinone in vitro have proven anti-inflammatory activities. On murine macrophages RAW 264.7 with a lipopolysaccharide (LPS)-induced inflammation model, FU and fucoxanthin isomers such as 9′-cis or 13′-fucoxanthin all displayed a significant dose-dependent inhibition of pro-inflammatory mediators IL6-IL-1, NO, and TNF-α [212]. Likewise, apo-9′-fucoxanthinone notably reduced levels of NO, ROS, TNF-α, and COX enzyme both in RAW 264.7 macrophages and zebrafish juveniles [213]. A study with different human colon and prostate cancer cell lines elucidated that besides the anti-inflammatory and antioxidant effect of β-carotene, it exerts a direct pro-apoptotic activity on cancerous cells by reducing the expression of caveolin-1 and inducing the activity of several caspases. This protein is heavily involved in cell cycle regulation, and its expression leads to increased protein kinase B levels, being both liable of cell proliferation. Conversely, caspases are signals for apoptosis. The authors were able to elucidate this significant pathway of cell growth inhibition, as this was observed in human colon and prostate cell lines that expressed caveolin-1 (HCT-116, PC-3), but not in those that do not produce it (Caco-2, LNCaP) [229].
3.3.2. Observation In Vivo
3.3.3. Observational and Epidemiological Studies
In the last decades, case-control and observational studies have also been carried out in humans to test the effectiveness of CA to extend life expectancy and other health-promoting effects such as reducing the risk of developing cancers, chronic inflammatory diseases, or cardiovascular diseases. Results on the possible chemopreventive effect of CA, especially of β-carotene, are mixed [231]. Nevertheless, this effectiveness has been reported in other studies. Various studies are available, for example, evaluating the potential health-promoting effects of LU. One of them analyzed the effect of LU supplementation in subjects from the Shanghai region with early symptoms of atherosclerosis. Albeit the study was carried out with a small sample (n = 65), it was observed that the levels of IL-6, MCP-1, and LDL-cholesterol were significantly lower [221]. In another study, food supplementation with β-carotene, lycopene, and lutein was provided to preterm infants. Although only C reactive was used as an inflammation marker, treated groups displayed significantly lower levels alongside improved retinal development in comparison with the control group [222]. The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study, which was carried out in 1994 with more than 25,000 (n = 29,133) median age male smokers, determined that intake of β-carotene and α-tocopherol supplements could increase the risk of lung cancer, after a ≤8 year follow-up [232]. Additionally, a 24-year follow-up of these subjects did not find a significant chemopreventive effect for supplementing β-carotene toward liver cancer incidence, but it did seem to exert a protective effect in diabetic subjects [233]. However, a recent prospective cohort study of a 30-year follow-up from these subjects determined a significant (p < 0.0001) correlation between CA serum levels and reduced all-cause mortality risk in the study quintiles that displayed higher CA in serum as a result of supplement intake, despite their advanced age and smoking habits [234]. These mixed results, also reported in other prospective cohort studies, show a general trend of a protective effect of CA toward cancer development and inflammation, of which research has focused extensively in β-carotene. However, the increased risks of lung cancer development observed in some studies could arguably be due to an excess of retinol in treated groups, as many studies used high-dosage CA supplements as treatment, while subjects may also intake these CA through diet [233]. Taking the case of the ATBC study, the β-carotene dose was of 20 mg, as much as three times the recommended dietary allowance of retinol [232]. Conversely, α-carotene, lycopene, and β-cryptoxanthin have been inversely correlated with developing lung cancer or at least showing a consistent chemopreventive effect [235]. Another study assessed serum CA levels from individuals from the US Third Nutrition and Health Examination Survey (NHANES III) [224], which evaluated health habits and analyzed the serum samples of the participants. In this prospective cohort study, α-carotene and β-cryptoxanthin also displayed effectiveness in lowering the risk of lung cancer development in smokers, but this effect was not apparent in non-smokers [225]. An extensive meta-analysis of human observational studies with a total sample size of more than 150,000 individuals (n = 174,067) assessed results from 13 studies, determining that provitamin A CA may exert a protective effect against cancer or cardiovascular mortality [236]. Yet, the authors noted that as mentioned, an excessive production of retinol because of supplementation may be responsible for the reported increased risks of lung cancer development in some case-control studies that considered these variables. It is noteworthy that the greatest meta-analysis up to date to our knowledge evaluated 34 observational studies with a total sample size of 592,479 participants and established correlations between intake or serum levels of α-carotene and lycopene but not β-carotene with lowered risk of developing prostate cancer [237]. These findings also noted that even if these carotenoids had an apparent chemopreventive activity, they were ineffective in preventing malignancy of prostate cancer once it was diagnosed. Altogether, albeit more extensive research with bigger sample sizes and the isolation of potential confusion factors is required, there is a great body of evidence suggesting that in controlled dose ranges, both provitamin A and non-provitamin carotenoids have chemopreventive effects on oxidative stress, inflammation, and cancer development through indirect and direct pathways.
This entry is adapted from the peer-reviewed paper 10.3390/md19040188
